
|
|||||||
|
Fusion Protein:HSPA1A-SLC9A3R1 |
Fusion Gene and Fusion Protein Summary |
 Fusion gene summary Fusion gene summary |
| Fusion partner gene information | Fusion gene name: HSPA1A-SLC9A3R1 | FusionPDB ID: 37857 | FusionGDB2.0 ID: 37857 | Hgene | Tgene | Gene symbol | HSPA1A | SLC9A3R1 | Gene ID | 3303 | 9368 |
| Gene name | heat shock protein family A (Hsp70) member 1A | SLC9A3 regulator 1 | |
| Synonyms | HEL-S-103|HSP70-1|HSP70-1A|HSP70-2|HSP70.1|HSP70.2|HSP70I|HSP72|HSPA1 | EBP50|NHERF|NHERF-1|NHERF1|NPHLOP2 | |
| Cytomap | 6p21.33 | 17q25.1 | |
| Type of gene | protein-coding | protein-coding | |
| Description | heat shock 70 kDa protein 1AHSP70-1/HSP70-2HSP70.1/HSP70.2Heat shock 70 kDa protein 1BHeat shock 70 kDa protein 2dnaK-type molecular chaperone HSP70-1epididymis secretory protein Li 103epididymis secretory sperm binding proteinheat shock 70 kDa pr | Na(+)/H(+) exchange regulatory cofactor NHE-RF1Na+/H+ exchange regulatory co-factorezrin-radixin-moesin binding phosphoprotein-50regulatory cofactor of Na(+)/H(+) exchangersolute carrier family 9 (sodium/hydrogen exchanger), isoform 3 regulatory facto | |
| Modification date | 20200327 | 20200313 | |
| UniProtAcc | P0DMV8 Main function of 5'-partner protein: FUNCTION: Molecular chaperone implicated in a wide variety of cellular processes, including protection of the proteome from stress, folding and transport of newly synthesized polypeptides, activation of proteolysis of misfolded proteins and the formation and dissociation of protein complexes. Plays a pivotal role in the protein quality control system, ensuring the correct folding of proteins, the re-folding of misfolded proteins and controlling the targeting of proteins for subsequent degradation. This is achieved through cycles of ATP binding, ATP hydrolysis and ADP release, mediated by co-chaperones. The co-chaperones have been shown to not only regulate different steps of the ATPase cycle, but they also have an individual specificity such that one co-chaperone may promote folding of a substrate while another may promote degradation. The affinity for polypeptides is regulated by its nucleotide bound state. In the ATP-bound form, it has a low affinity for substrate proteins. However, upon hydrolysis of the ATP to ADP, it undergoes a conformational change that increases its affinity for substrate proteins. It goes through repeated cycles of ATP hydrolysis and nucleotide exchange, which permits cycles of substrate binding and release. The co-chaperones are of three types: J-domain co-chaperones such as HSP40s (stimulate ATPase hydrolysis by HSP70), the nucleotide exchange factors (NEF) such as BAG1/2/3 (facilitate conversion of HSP70 from the ADP-bound to the ATP-bound state thereby promoting substrate release), and the TPR domain chaperones such as HOPX and STUB1 (PubMed:24012426, PubMed:26865365, PubMed:24318877). Maintains protein homeostasis during cellular stress through two opposing mechanisms: protein refolding and degradation. Its acetylation/deacetylation state determines whether it functions in protein refolding or protein degradation by controlling the competitive binding of co-chaperones HOPX and STUB1. During the early stress response, the acetylated form binds to HOPX which assists in chaperone-mediated protein refolding, thereafter, it is deacetylated and binds to ubiquitin ligase STUB1 that promotes ubiquitin-mediated protein degradation (PubMed:27708256). Regulates centrosome integrity during mitosis, and is required for the maintenance of a functional mitotic centrosome that supports the assembly of a bipolar mitotic spindle (PubMed:27137183). Enhances STUB1-mediated SMAD3 ubiquitination and degradation and facilitates STUB1-mediated inhibition of TGF-beta signaling (PubMed:24613385). Essential for STUB1-mediated ubiquitination and degradation of FOXP3 in regulatory T-cells (Treg) during inflammation (PubMed:23973223). Negatively regulates heat shock-induced HSF1 transcriptional activity during the attenuation and recovery phase period of the heat shock response (PubMed:9499401). Involved in the clearance of misfolded PRDM1/Blimp-1 proteins. Sequesters them in the cytoplasm and promotes their association with SYNV1/HRD1, leading to proteasomal degradation (PubMed:28842558). {ECO:0000269|PubMed:22528486, ECO:0000269|PubMed:23973223, ECO:0000269|PubMed:24318877, ECO:0000269|PubMed:24613385, ECO:0000269|PubMed:27137183, ECO:0000269|PubMed:27708256, ECO:0000269|PubMed:28842558, ECO:0000269|PubMed:9499401, ECO:0000303|PubMed:24012426, ECO:0000303|PubMed:26865365}.; FUNCTION: (Microbial infection) In case of rotavirus A infection, serves as a post-attachment receptor for the virus to facilitate entry into the cell. {ECO:0000269|PubMed:16537599}. | . | |
| Ensembl transtripts involved in fusion gene | ENST ids | ENST00000375651, ENST00000458062, ENST00000608703, ENST00000383389, ENST00000400040, ENST00000422919, ENST00000430065, ENST00000433487, ENST00000441618, ENST00000449876, ENST00000452298, | ENST00000413388, ENST00000262613, |
| Fusion gene scores for assessment (based on all fusion genes of FusionGDB 2.0) | * DoF score | 7 X 8 X 2=112 | 9 X 12 X 6=648 |
| # samples | 7 | 13 | |
| ** MAII score | log2(7/112*10)=-0.678071905112638 possibly effective Gene in Pan-Cancer Fusion Genes (peGinPCFGs). DoF>8 and MAII<0 | log2(13/648*10)=-2.31748218985617 possibly effective Gene in Pan-Cancer Fusion Genes (peGinPCFGs). DoF>8 and MAII<0 | |
| Fusion gene context | PubMed: HSPA1A [Title/Abstract] AND SLC9A3R1 [Title/Abstract] AND fusion [Title/Abstract] | ||
| Fusion neoantigen context | PubMed: HSPA1A [Title/Abstract] AND SLC9A3R1 [Title/Abstract] AND neoantigen [Title/Abstract] | ||
| Most frequent breakpoint (based on all fusion genes of FusionGDB 2.0) | HSPA1A(31785221)-SLC9A3R1(72745398), # samples:1 HSPA1A(31797415)-SLC9A3R1(72745398), # samples:1 | ||
| Anticipated loss of major functional domain due to fusion event. | HSPA1A-SLC9A3R1 seems lost the major protein functional domain in Hgene partner, which is a CGC by not retaining the major functional domain in the partially deleted in-frame ORF. HSPA1A-SLC9A3R1 seems lost the major protein functional domain in Hgene partner, which is a essential gene by not retaining the major functional domain in the partially deleted in-frame ORF. | ||
| * DoF score (Degree of Frequency) = # partners X # break points X # cancer types ** MAII score (Major Active Isofusion Index) = log2(# samples/DoF score*10) |
 Gene ontology of each fusion partner gene with evidence of Inferred from Direct Assay (IDA) from Entrez Gene ontology of each fusion partner gene with evidence of Inferred from Direct Assay (IDA) from Entrez |
| Partner | Gene | GO ID | GO term | PubMed ID |
| Hgene | HSPA1A | GO:0006402 | mRNA catabolic process | 10205060 |
| Hgene | HSPA1A | GO:0006986 | response to unfolded protein | 10859165 |
| Hgene | HSPA1A | GO:0031396 | regulation of protein ubiquitination | 16809764 |
| Hgene | HSPA1A | GO:0031397 | negative regulation of protein ubiquitination | 12150907 |
| Hgene | HSPA1A | GO:0032436 | positive regulation of proteasomal ubiquitin-dependent protein catabolic process | 24613385 |
| Hgene | HSPA1A | GO:0033120 | positive regulation of RNA splicing | 20625543 |
| Hgene | HSPA1A | GO:0034605 | cellular response to heat | 24061851 |
| Hgene | HSPA1A | GO:0042026 | protein refolding | 15603737|21231916 |
| Hgene | HSPA1A | GO:0046034 | ATP metabolic process | 23921388 |
| Hgene | HSPA1A | GO:0050821 | protein stabilization | 21909508 |
| Hgene | HSPA1A | GO:0051131 | chaperone-mediated protein complex assembly | 10811660 |
| Hgene | HSPA1A | GO:0090084 | negative regulation of inclusion body assembly | 15603737|21231916 |
| Hgene | HSPA1A | GO:0097201 | negative regulation of transcription from RNA polymerase II promoter in response to stress | 9499401 |
| Hgene | HSPA1A | GO:1901029 | negative regulation of mitochondrial outer membrane permeabilization involved in apoptotic signaling pathway | 20625543 |
| Hgene | HSPA1A | GO:1902236 | negative regulation of endoplasmic reticulum stress-induced intrinsic apoptotic signaling pathway | 12150907|20625543 |
| Hgene | HSPA1A | GO:1902380 | positive regulation of endoribonuclease activity | 20625543 |
| Tgene | SLC9A3R1 | GO:0008285 | negative regulation of cell proliferation | 20012548 |
| Tgene | SLC9A3R1 | GO:0070373 | negative regulation of ERK1 and ERK2 cascade | 20012548 |
| Tgene | SLC9A3R1 | GO:2001244 | positive regulation of intrinsic apoptotic signaling pathway | 20012548 |
 Four levels of functional features of fusion genes Four levels of functional features of fusion genesGo to FGviewer search page for the most frequent breakpoint (https://ccsmweb.uth.edu/FGviewer/chr6:31785221/chr17:72745398) - FGviewer provides the online visualization of the retention search of the protein functional features across DNA, RNA, protein, and pathological levels. - How to search 1. Put your fusion gene symbol. 2. Press the tab key until there will be shown the breakpoint information filled. 4. Go down and press 'Search' tab twice. 4. Go down to have the hyperlink of the search result. 5. Click the hyperlink. 6. See the FGviewer result for your fusion gene. |
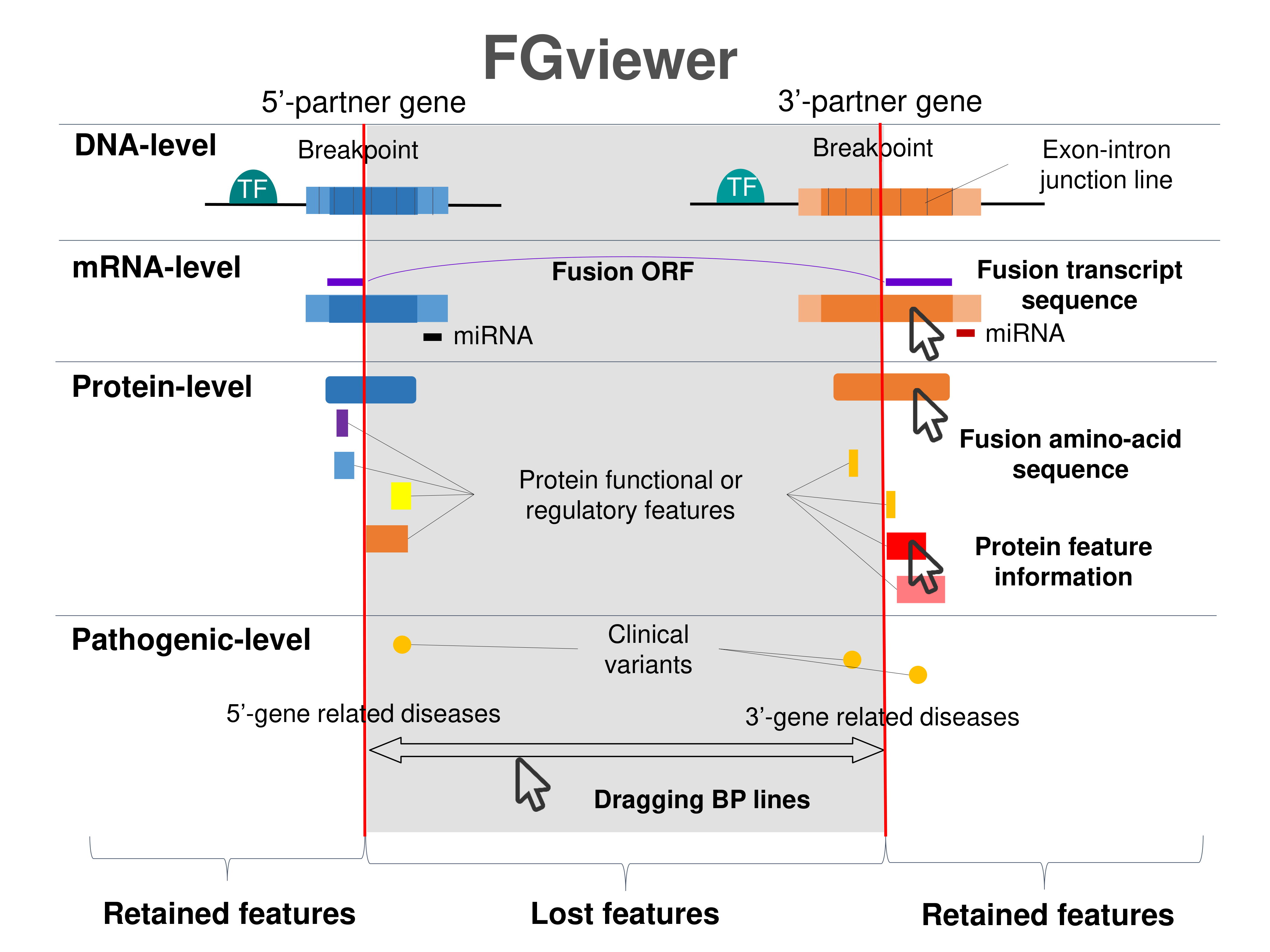 |
 Retention analysis results of each fusion partner protein across 39 protein features of UniProt such as six molecule processing features, 13 region features, four site features, six amino acid modification features, two natural variation features, five experimental info features, and 3 secondary structure features, are available here. Retention analysis results of each fusion partner protein across 39 protein features of UniProt such as six molecule processing features, 13 region features, four site features, six amino acid modification features, two natural variation features, five experimental info features, and 3 secondary structure features, are available here. |
 Fusion gene breakpoints across HSPA1A (5'-gene) Fusion gene breakpoints across HSPA1A (5'-gene)* Click on the image to open the UCSC genome browser with custom track showing this image in a new window. |
 |
 Fusion gene breakpoints across SLC9A3R1 (3'-gene) Fusion gene breakpoints across SLC9A3R1 (3'-gene)* Click on the image to open the UCSC genome browser with custom track showing this image in a new window. |
 |
Top |
Fusion Amino Acid Sequences |
 Fusion information from ORFfinder translation from full-length transcript sequence from FusionPDB. Fusion information from ORFfinder translation from full-length transcript sequence from FusionPDB. |
| Henst | Tenst | Hgene | Hchr | Hbp | Hstrand | Tgene | Tchr | Tbp | Tstrand | Seq length (transcript) | BP loci (transcript) | Predicted start (transcript) | Predicted stop (transcript) | Seq length (amino acids) |
| ENST00000375651 | HSPA1A | chr6 | 31785221 | + | ENST00000262613 | SLC9A3R1 | chr17 | 72745398 | + | 1857 | 496 | 551 | 1159 | 202 |
| ENST00000458062 | HSPA1A | chr6 | 31785221 | + | ENST00000262613 | SLC9A3R1 | chr17 | 72745398 | + | 1894 | 533 | 588 | 1196 | 202 |
| ENST00000608703 | HSPA1A | chr6 | 31785221 | + | ENST00000262613 | SLC9A3R1 | chr17 | 72745398 | + | 2152 | 791 | 846 | 1454 | 202 |
 DeepORF prediction of the coding potential based on the fusion transcript sequence of in-frame fusion genes. DeepORF is a coding potential classifier based on convolutional neural network by comparing the real Ribo-seq data. If the no-coding score < 0.5 and coding score > 0.5, then the in-frame fusion transcript is predicted as being likely translated. DeepORF prediction of the coding potential based on the fusion transcript sequence of in-frame fusion genes. DeepORF is a coding potential classifier based on convolutional neural network by comparing the real Ribo-seq data. If the no-coding score < 0.5 and coding score > 0.5, then the in-frame fusion transcript is predicted as being likely translated. |
| Henst | Tenst | Hgene | Hchr | Hbp | Hstrand | Tgene | Tchr | Tbp | Tstrand | No-coding score | Coding score |
| ENST00000375651 | ENST00000262613 | HSPA1A | chr6 | 31785221 | + | SLC9A3R1 | chr17 | 72745398 | + | 0.006331588 | 0.9936684 |
| ENST00000458062 | ENST00000262613 | HSPA1A | chr6 | 31785221 | + | SLC9A3R1 | chr17 | 72745398 | + | 0.012538657 | 0.9874614 |
| ENST00000608703 | ENST00000262613 | HSPA1A | chr6 | 31785221 | + | SLC9A3R1 | chr17 | 72745398 | + | 0.009763063 | 0.99023694 |
 Predicted full-length fusion amino acid sequences. For individual full-length fusion transcript sequence from FusionPDB, we ran ORFfinder and chose the longest ORF among all the predicted ones. Predicted full-length fusion amino acid sequences. For individual full-length fusion transcript sequence from FusionPDB, we ran ORFfinder and chose the longest ORF among all the predicted ones. |
Get the fusion protein sequences from here. |
| Fusion protein sequence information is available in the fasta format. >FusionGDB ID_FusionGDB isoform ID_FGname_Hgene_Hchr_Hbp_Henst_Tgene_Tchr_Tbp_Tenst_length(fusion AA) seq_BP |
Top |
Fusion Protein Breakpoint Sequences for HSPA1A-SLC9A3R1 |
 +/-13 AA sequence from the breakpoints of the fusion protein sequences. +/-13 AA sequence from the breakpoints of the fusion protein sequences. |
| Hgene | Hchr | Hbp | Tgene | Tchr | Tbp | Length(fusion protein) | BP in fusion protein | Peptide |
Top |
Potential FusionNeoAntigen Information of HSPA1A-SLC9A3R1 in HLA I |
 Multiple sequence alignments of the potential FusionNeoAntigens per fusion breakpoints. If the MSA is empty, then it means that there were predicted fusion neoantigens in this fusion breakpoint, but those predicted fusion neoantigens were not across the breakpoint, which is not fusion-specific. Multiple sequence alignments of the potential FusionNeoAntigens per fusion breakpoints. If the MSA is empty, then it means that there were predicted fusion neoantigens in this fusion breakpoint, but those predicted fusion neoantigens were not across the breakpoint, which is not fusion-specific. |
 Potential FusionNeoAntigen Information Potential FusionNeoAntigen Information* We used NetMHCpan v4.1 (%rank<0.5) and deepHLApan v1.1 (immunogenic score>0.5) |
| Fusion gene | Hchr | Hbp | Tgene | Tchr | Tbp | HLA I | FusionNeoAntigen peptide | Binding score | Immunogenic score | Neoantigen start (at BP 13) | Neoantigen end (at BP 13) |
Top |
Potential FusionNeoAntigen Information of HSPA1A-SLC9A3R1 in HLA II |
 Multiple sequence alignments of the potential FusionNeoAntigens per fusion breakpoints. If the MSA is empty, then it means that there were predicted fusion neoantigens in this fusion breakpoint, but those predicted fusion neoantigens were not across the breakpoint, which is not fusion-specific. Multiple sequence alignments of the potential FusionNeoAntigens per fusion breakpoints. If the MSA is empty, then it means that there were predicted fusion neoantigens in this fusion breakpoint, but those predicted fusion neoantigens were not across the breakpoint, which is not fusion-specific. |
 Potential FusionNeoAntigen Information Potential FusionNeoAntigen Information * We used NetMHCIIpan v4.1 (%rank<0.5). |
| Fusion gene | Hchr | Hbp | Tgene | Tchr | Tbp | HLA II | FusionNeoAntigen peptide | Neoantigen start (at BP 13) | Neoantigen end (at BP 13) |
Top |
Fusion breakpoint peptide structures of HSPA1A-SLC9A3R1 |
 3D structures of the fusion breakpoint peptide of 14AA sequence that have potential fusion neoantigens 3D structures of the fusion breakpoint peptide of 14AA sequence that have potential fusion neoantigens* The minimum length of the amino acid sequence in RoseTTAFold is 14AA. Here, we predicted the 14AA fusion protein breakpoint sequence not the fusion neoantigen peptide, which is shorter than 14 AA. |
Top |
Filtering FusionNeoAntigens Through Checking the Interaction with HLAs in 3D of HSPA1A-SLC9A3R1 |
 Virtual screening between 25 HLAs (from PDB) and FusionNeoAntigens Virtual screening between 25 HLAs (from PDB) and FusionNeoAntigens* We used Glide to predict the interaction between HLAs and neoantigens. |
| HLA allele | PDB ID | File name | BPseq | Docking score | Glide score |
Top |
Vaccine Design for the FusionNeoAntigens of HSPA1A-SLC9A3R1 |
 mRNA and peptide sequences of FusionNeoAntigens that have potential interaction with HLA-Is. mRNA and peptide sequences of FusionNeoAntigens that have potential interaction with HLA-Is. |
| Fusion gene | Hchr | Hbp | Tchr | Tbp | Start in +/-13AA | End in +/-13AA | FusionNeoAntigen peptide sequence | FusionNeoAntigen RNA sequence |
 mRNA and peptide sequences of FusionNeoAntigens that have potential interaction with HLA-IIs. mRNA and peptide sequences of FusionNeoAntigens that have potential interaction with HLA-IIs. |
| Fusion gene | Hchr | Hbp | Tchr | Tbp | Start in +/-13AA | End in +/-13AA | FusionNeoAntigen peptide | FusionNEoAntigen RNA sequence |
Top |
Information of the samples that have these potential fusion neoantigens of HSPA1A-SLC9A3R1 |
 These samples were reported as having these fusion breakpoints. For individual breakpoints, we checked the open reading frames considering multiple gene isoforms and chose the in-frame fusion genes only. Then, we made fusion protein sequences and predicted the fusion neoantigens. These fusion-positive samples may have these potential fusion neoantigens. These samples were reported as having these fusion breakpoints. For individual breakpoints, we checked the open reading frames considering multiple gene isoforms and chose the in-frame fusion genes only. Then, we made fusion protein sequences and predicted the fusion neoantigens. These fusion-positive samples may have these potential fusion neoantigens. |
| Cancer type | Fusion gene | Hchr | Hbp | Henst | Tchr | Tbp | Tenst | Sample |
Top |
Potential target of CAR-T therapy development for HSPA1A-SLC9A3R1 |
 Predicted 3D structure. We used RoseTTAFold. Predicted 3D structure. We used RoseTTAFold. |
 Retention analysis result of each fusion partner protein across 39 protein features of UniProt such as six molecule processing features, 13 region features, four site features, six amino acid modification features, two natural variation features, five experimental info features, and 3 secondary structure features. Here, to provide the retention of the transmembrane domain, we only show the protein feature retention information of those transmembrane features Retention analysis result of each fusion partner protein across 39 protein features of UniProt such as six molecule processing features, 13 region features, four site features, six amino acid modification features, two natural variation features, five experimental info features, and 3 secondary structure features. Here, to provide the retention of the transmembrane domain, we only show the protein feature retention information of those transmembrane features* Minus value of BPloci means that the break point is located before the CDS. |
| - In-frame and retained 'Transmembrane'. |
| Partner | Gene | Hbp | Tbp | ENST | Strand | BPexon | TotalExon | Protein feature loci | *BPloci | TotalLen | Protein feature | Protein feature note |
 Subcellular localization prediction of the transmembrane domain retained fusion proteins Subcellular localization prediction of the transmembrane domain retained fusion proteins* We used DeepLoc 1.0. The order of the X-axis of the barplot is as follows: Entry_ID, Localization, Type, Nucleus, Cytoplasm, Extracellular, Mitochondrion, Cell_membrane, Endoplasmic_reticulum, Plastid, Golgi.apparatus, Lysosome.Vacuole, Peroxisome. Y-axis is the output score of DeepLoc. Clicking the image will open a new tab with a large image. |
| Hgene | Hchr | Hbp | Henst | Tgene | Tchr | Tbp | Tenst | DeepLoc result |
Top |
Related Drugs to HSPA1A-SLC9A3R1 |
 Drugs used for this fusion-positive patient. Drugs used for this fusion-positive patient. (Manual curation of PubMed, 04-30-2022 + MyCancerGenome) |
| Hgene | Tgene | Drug | Source | PMID |
Top |
Related Diseases to HSPA1A-SLC9A3R1 |
 Diseases that have this fusion gene. Diseases that have this fusion gene. (Manual curation of PubMed, 04-30-2022 + MyCancerGenome) |
| Hgene | Tgene | Disease | Source | PMID |
 Diseases associated with fusion partners. Diseases associated with fusion partners. (DisGeNet 4.0) |
| Partner | Gene | Disease ID | Disease name | # pubmeds | Source |

