| UTHEALTH HOME ABOUT SBMI A-Z WEBMAIL INSIDE THE UNIVERSITY |

|
|||||||
|
Kinase Fusion Gene:HSP90AA1_RPS6KA3 |
Kinase Fusion Protein Summary |
 Kinase Fusion gene summary Kinase Fusion gene summary |
| Kinase Fusion partner gene information | Kinase Fusion gene name: HSP90AA1_RPS6KA3 | KinaseFusionDB ID: KFG2759 | FusionGDB2.0 ID: KFG2759 | Hgene | Tgene | Gene symbol | HSP90AA1 | RPS6KA3 | Gene ID | 3320 | 6197 | |
| Gene name | heat shock protein 90 alpha family class A member 1 | ribosomal protein S6 kinase A3 | ||||||||||
| Synonyms | EL52|HEL-S-65p|HSP86|HSP89A|HSP90A|HSP90N|HSPC1|HSPCA|HSPCAL1|HSPCAL4|HSPN|Hsp103|Hsp89|Hsp90|LAP-2|LAP2 | CLS|HU-3|ISPK-1|MAPKAPK1B|MRX19|RSK|RSK2|S6K-alpha3|XLID19|p90-RSK2|pp90RSK2 | ||||||||||
| Cytomap | 14q32.31 | Xp22.12 | ||||||||||
| Type of gene | protein-coding | protein-coding | ||||||||||
| Description | heat shock protein HSP 90-alphaHSP 86LPS-associated protein 2epididymis luminal secretory protein 52epididymis secretory sperm binding protein Li 65pheat shock 86 kDaheat shock 90kD protein 1, alphaheat shock 90kD protein 1, alpha-like 4heat shock | ribosomal protein S6 kinase alpha-3MAP kinase-activated protein kinase 1bMAPK-activated protein kinase 1bMAPKAP kinase 1bMAPKAPK-1bRSK-2S6K-alpha-3epididymis secretory sperm binding proteininsulin-stimulated protein kinase 1p90-RSK 3ribosomal S6 | ||||||||||
| Modification date | 20240407 | 20240403 | ||||||||||
| UniProtAcc | P07900 | P51812 | ||||||||||
| Ensembl transtripts involved in fusion gene | ENST ids | ENST00000216281, ENST00000334701, ENST00000441629, ENST00000558600, | ENST00000379565, ENST00000544447, ENST00000379548, ENST00000540702, ENST00000479809, | |||||||||
| Context (manual curation of fusion genes in KinaseFusionDB) | PubMed: HSP90AA1 [Title/Abstract] AND RPS6KA3 [Title/Abstract] AND fusion [Title/Abstract] | |||||||||||
| Most frequent breakpoint (based on all fusion genes of FusionGDB 2.0) | ||||||||||||
 Gene ontology of each fusion partner gene with evidence of Inferred from Direct Assay (IDA) from Entrez Gene ontology of each fusion partner gene with evidence of Inferred from Direct Assay (IDA) from Entrez |
| Partner | Gene | GO ID | GO term | PubMed ID |
| Hgene | HSP90AA1 | GO:0001934 | positive regulation of protein phosphorylation | 19363271 |
| Hgene | HSP90AA1 | GO:0002218 | activation of innate immune response | 20628368 |
| Hgene | HSP90AA1 | GO:0002230 | positive regulation of defense response to virus by host | 20628368 |
| Hgene | HSP90AA1 | GO:0007004 | telomere maintenance via telomerase | 10197982 |
| Hgene | HSP90AA1 | GO:0031396 | regulation of protein ubiquitination | 16809764 |
| Hgene | HSP90AA1 | GO:0032273 | positive regulation of protein polymerization | 19363271 |
| Hgene | HSP90AA1 | GO:0032728 | positive regulation of interferon-beta production | 20628368 |
| Hgene | HSP90AA1 | GO:0042981 | regulation of apoptotic process | 25609812 |
| Hgene | HSP90AA1 | GO:0045040 | protein insertion into mitochondrial outer membrane | 15644312 |
| Hgene | HSP90AA1 | GO:0051131 | chaperone-mediated protein complex assembly | 15644312 |
| Hgene | HSP90AA1 | GO:0051973 | positive regulation of telomerase activity | 10197982 |
| Hgene | HSP90AA1 | GO:0098586 | cellular response to virus | 25609812 |
| Hgene | HSP90AA1 | GO:1902949 | positive regulation of tau-protein kinase activity | 19363271 |
| Hgene | HSP90AA1 | GO:1905323 | telomerase holoenzyme complex assembly | 10197982 |
| Tgene | RPS6KA3 | GO:0043154 | negative regulation of cysteine-type endopeptidase activity involved in apoptotic process | 18402937 |
 Kinase Fusion gene breakpoints across HSP90AA1 (5'-gene) Kinase Fusion gene breakpoints across HSP90AA1 (5'-gene)* Click on the image to open the UCSC genome browser with custom track showing this image in a new window. |
 Kinase Fusion gene breakpoints across RPS6KA3 (3'-gene) Kinase Fusion gene breakpoints across RPS6KA3 (3'-gene)* Click on the image to open the UCSC genome browser with custom track showing this image in a new window. |
Top |
Kinase Fusion Gene Sample Information |
 Kinase Fusion gene information. Kinase Fusion gene information. |
 Kinase Fusion gene information from four resources (ChiTars 5.0, ChimerDB 4.0, COSMIC, and CCLE) Kinase Fusion gene information from four resources (ChiTars 5.0, ChimerDB 4.0, COSMIC, and CCLE)* All genome coordinats were lifted-over on hg19. * Click on the break point to see the gene structure around the break point region using the UCSC Genome Browser. |
| Source | Sample | Hgene | Hchr | Hbp | Tgene | Tchr | Tbp |
| CCLE | Omm2.5 | HSP90AA1 | chr14 | 102605587 | RPS6KA3 | chrX | 20252932 |
Top |
Kinase Fusion ORF Analysis |
 Kinase Fusion information from ORFfinder translation from full-length transcript sequence from KinaseFusionDB. Kinase Fusion information from ORFfinder translation from full-length transcript sequence from KinaseFusionDB. |
| Henst | Tenst | Hgene | Hchr | Hbp | Tgene | Tchr | Tbp | Seq length (transcript) | Seq length (amino acids) |
| ENST00000334701 | ENST00000379565 | HSP90AA1 | chr14 | 102605587 | RPS6KA3 | chrX | 20252932 | 8078 | 712 |
Top |
Kinase Fusion Amino Acid Sequences |
 For individual full-length fusion transcript sequence from KinaseFusionDB, we ran ORFfinder and chose the longest ORF among the all predicted ones. For individual full-length fusion transcript sequence from KinaseFusionDB, we ran ORFfinder and chose the longest ORF among the all predicted ones. |
| >Henst_Tenst_Hgene_Hchr_Hbp_Tgene_Tchr_Tbp_length(fusion AA)_AAseq >ENST00000334701_ENST00000379565_HSP90AA1_chr14_102605587_RPS6KA3_chrX_20252932_length(amino acids)=712 MDEPMGEEEINPQTEEVSIKEIAITHHVKEGHEKADPSQFELLKVLGQGSFGKVFLVKKISGSDARQLYAMKVLKKATLKVRDRVRTKME RDILVEVNHPFIVKLHYAFQTEGKLYLILDFLRGGDLFTRLSKEVMFTEEDVKFYLAELALALDHLHSLGIIYRDLKPENILLDEEGHIK LTDFGLSKESIDHEKKAYSFCGTVEYMAPEVVNRRGHTQSADWWSFGVLMFEMLTGTLPFQGKDRKETMTMILKAKLGMPQFLSPEAQSL LRMLFKRNPANRLGAGPDGVEEIKRHSFFSTIDWNKLYRREIHPPFKPATGRPEDTFYFDPEFTAKTPKDSPGIPPSANAHQLFRGFSFV AITSDDESQAMQTVGVHSIVQQLHRNSIQFTDGYEVKEDIGVGSYSVCKRCIHKATNMEFAVKIIDKSKRDPTEEIEILLRYGQHPNIIT LKDVYDDGKYVYVVTELMKGGELLDKILRQKFFSEREASAVLFTITKTVEYLHAQGVVHRDLKPSNILYVDESGNPESIRICDFGFAKQL RAENGLLMTPCYTANFVAPEVLKRQGYDAACDIWSLGVLLYTMLTGYTPFANGPDDTPEEILARIGSGKFSLSGGYWNSVSDTAKDLVSK -------------------------------------------------------------- |
Multiple Sequence Alignment of All Fusion Protein Isoforms |
Top |
Kinase Fusion Protein Functional Features |
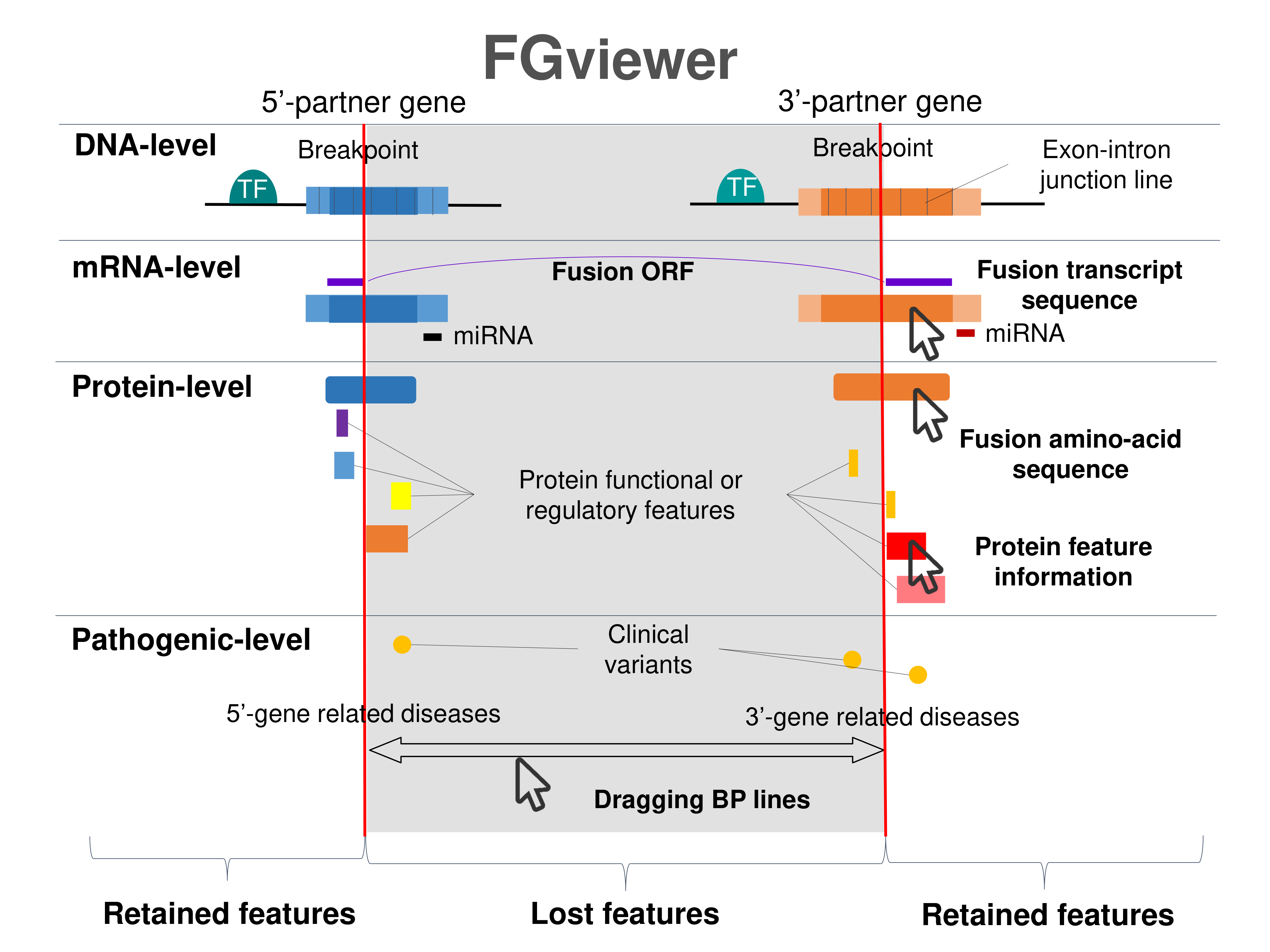
 Four levels of functional features of fusion genes Four levels of functional features of fusion genesGo to FGviewer search page for the most frequent breakpoint (https://ccsmweb.uth.edu/FGviewer/chr14:/chrX:) - FGviewer provides the online visualization of the retention search of the protein functional features across DNA, RNA, protein, and pathological levels. - How to search 1. Put your fusion gene symbol. 2. Press the tab key until there will be shown the breakpoint information filled. 4. Go down and press 'Search' tab twice. 4. Go down to have the hyperlink of the search result. 5. Click the hyperlink. 6. See the FGviewer result for your fusion gene. |
 |
 Main function of each fusion partner protein. (from UniProt) Main function of each fusion partner protein. (from UniProt) |
| Hgene | Tgene |
| HSP90AA1 | RPS6KA3 |
| FUNCTION: Molecular chaperone that promotes the maturation, structural maintenance and proper regulation of specific target proteins involved for instance in cell cycle control and signal transduction. Undergoes a functional cycle that is linked to its ATPase activity which is essential for its chaperone activity. This cycle probably induces conformational changes in the client proteins, thereby causing their activation. Interacts dynamically with various co-chaperones that modulate its substrate recognition, ATPase cycle and chaperone function (PubMed:11274138, PubMed:15577939, PubMed:15937123, PubMed:27353360, PubMed:29127155, PubMed:12526792). Engages with a range of client protein classes via its interaction with various co-chaperone proteins or complexes, that act as adapters, simultaneously able to interact with the specific client and the central chaperone itself (PubMed:29127155). Recruitment of ATP and co-chaperone followed by client protein forms a functional chaperone. After the completion of the chaperoning process, properly folded client protein and co-chaperone leave HSP90 in an ADP-bound partially open conformation and finally, ADP is released from HSP90 which acquires an open conformation for the next cycle (PubMed:27295069, PubMed:26991466). Plays a critical role in mitochondrial import, delivers preproteins to the mitochondrial import receptor TOMM70 (PubMed:12526792). Apart from its chaperone activity, it also plays a role in the regulation of the transcription machinery. HSP90 and its co-chaperones modulate transcription at least at three different levels (PubMed:25973397). In the first place, they alter the steady-state levels of certain transcription factors in response to various physiological cues(PubMed:25973397). Second, they modulate the activity of certain epigenetic modifiers, such as histone deacetylases or DNA methyl transferases, and thereby respond to the change in the environment (PubMed:25973397). Third, they participate in the eviction of histones from the promoter region of certain genes and thereby turn on gene expression (PubMed:25973397). Binds bacterial lipopolysaccharide (LPS) and mediates LPS-induced inflammatory response, including TNF secretion by monocytes (PubMed:11276205). Antagonizes STUB1-mediated inhibition of TGF-beta signaling via inhibition of STUB1-mediated SMAD3 ubiquitination and degradation (PubMed:24613385). Mediates the association of TOMM70 with IRF3 or TBK1 in mitochondrial outer membrane which promotes host antiviral response (PubMed:20628368, PubMed:25609812). {ECO:0000269|PubMed:11274138, ECO:0000269|PubMed:11276205, ECO:0000269|PubMed:12526792, ECO:0000269|PubMed:15577939, ECO:0000269|PubMed:15937123, ECO:0000269|PubMed:20628368, ECO:0000269|PubMed:24613385, ECO:0000269|PubMed:25609812, ECO:0000269|PubMed:27353360, ECO:0000269|PubMed:29127155, ECO:0000303|PubMed:25973397, ECO:0000303|PubMed:26991466, ECO:0000303|PubMed:27295069}.; FUNCTION: (Microbial infection) Seems to interfere with N.meningitidis NadA-mediated invasion of human cells. Decreasing HSP90 levels increases adhesion and entry of E.coli expressing NadA into human Chang cells; increasing its levels leads to decreased adhesion and invasion. {ECO:0000305|PubMed:22066472}. | FUNCTION: Serine/threonine-protein kinase that acts downstream of ERK (MAPK1/ERK2 and MAPK3/ERK1) signaling and mediates mitogenic and stress-induced activation of the transcription factors CREB1, ETV1/ER81 and NR4A1/NUR77, regulates translation through RPS6 and EIF4B phosphorylation, and mediates cellular proliferation, survival, and differentiation by modulating mTOR signaling and repressing pro-apoptotic function of BAD and DAPK1 (PubMed:9770464, PubMed:16223362, PubMed:17360704, PubMed:16213824). In fibroblast, is required for EGF-stimulated phosphorylation of CREB1 and histone H3 at 'Ser-10', which results in the subsequent transcriptional activation of several immediate-early genes (PubMed:9770464, PubMed:10436156). In response to mitogenic stimulation (EGF and PMA), phosphorylates and activates NR4A1/NUR77 and ETV1/ER81 transcription factors and the cofactor CREBBP (PubMed:16223362). Upon insulin-derived signal, acts indirectly on the transcription regulation of several genes by phosphorylating GSK3B at 'Ser-9' and inhibiting its activity (PubMed:8250835). Phosphorylates RPS6 in response to serum or EGF via an mTOR-independent mechanism and promotes translation initiation by facilitating assembly of the preinitiation complex (PubMed:17360704). In response to insulin, phosphorylates EIF4B, enhancing EIF4B affinity for the EIF3 complex and stimulating cap-dependent translation (PubMed:18508509, PubMed:18813292). Is involved in the mTOR nutrient-sensing pathway by directly phosphorylating TSC2 at 'Ser-1798', which potently inhibits TSC2 ability to suppress mTOR signaling, and mediates phosphorylation of RPTOR, which regulates mTORC1 activity and may promote rapamycin-sensitive signaling independently of the PI3K/AKT pathway (PubMed:18722121). Mediates cell survival by phosphorylating the pro-apoptotic proteins BAD and DAPK1 and suppressing their pro-apoptotic function (PubMed:16213824). Promotes the survival of hepatic stellate cells by phosphorylating CEBPB in response to the hepatotoxin carbon tetrachloride (CCl4) (PubMed:18508509, PubMed:18813292). Is involved in cell cycle regulation by phosphorylating the CDK inhibitor CDKN1B, which promotes CDKN1B association with 14-3-3 proteins and prevents its translocation to the nucleus and inhibition of G1 progression (By similarity). In LPS-stimulated dendritic cells, is involved in TLR4-induced macropinocytosis, and in myeloma cells, acts as effector of FGFR3-mediated transformation signaling, after direct phosphorylation at Tyr-529 by FGFR3 (By similarity). Negatively regulates EGF-induced MAPK1/3 phosphorylation via phosphorylation of SOS1 (By similarity). Phosphorylates SOS1 at 'Ser-1134' and 'Ser-1161' that create YWHAB and YWHAE binding sites and which contribute to the negative regulation of MAPK1/3 phosphorylation (By similarity). Phosphorylates EPHA2 at 'Ser-897', the RPS6KA-EPHA2 signaling pathway controls cell migration (PubMed:26158630). Acts as a regulator of osteoblast differentiation by mediating phosphorylation of ATF4, thereby promoting ATF4 transactivation activity (By similarity). {ECO:0000250|UniProtKB:P18654, ECO:0000269|PubMed:10436156, ECO:0000269|PubMed:16213824, ECO:0000269|PubMed:16223362, ECO:0000269|PubMed:17360704, ECO:0000269|PubMed:18722121, ECO:0000269|PubMed:26158630, ECO:0000269|PubMed:8250835, ECO:0000269|PubMed:9770464, ECO:0000303|PubMed:18508509, ECO:0000303|PubMed:18813292}. |
 Retention analysis result of each fusion partner protein across 39 protein features of UniProt such as six molecule processing features, 13 region features, four site features, six amino acid modification features, two natural variation features, five experimental info features, and 3 secondary structure features. Here, because of limited space for viewing, we only show the protein feature retention information belong to the 13 regional features. All retention annotation result can be downloaded at * Minus value of BPloci means that the break pointn is located before the CDS. Retention analysis result of each fusion partner protein across 39 protein features of UniProt such as six molecule processing features, 13 region features, four site features, six amino acid modification features, two natural variation features, five experimental info features, and 3 secondary structure features. Here, because of limited space for viewing, we only show the protein feature retention information belong to the 13 regional features. All retention annotation result can be downloaded at * Minus value of BPloci means that the break pointn is located before the CDS. |
 - Retained domain in the 5'-partner of fusion protein (protein functional feature from UniProt). - Retained domain in the 5'-partner of fusion protein (protein functional feature from UniProt). |
| Partner | Hgeneene | Hbp | Tgeneene | Tbp | ENST | BPexon | TotalExon | Protein feature loci | BPloci | TotalLen | Feature | Note |
 - Retained domain in the 3'-partner of fusion protein (protein functional feature from UniProt). - Retained domain in the 3'-partner of fusion protein (protein functional feature from UniProt). |
| Partner | Hgeneene | Hbp | Tgeneene | Tbp | ENST | BPexon | TotalExon | Protein feature loci | BPloci | TotalLen | Feature | Note |
| Tgene | HSP90AA1 | 102605587 | RPS6KA3 | 20252932 | ENST00000334701 | 0 | 22 | 328_397 | 23 | 741 | Domain | Note=AGC-kinase C-terminal;Ontology_term=ECO:0000255;evidence=ECO:0000255|PROSITE-ProRule:PRU00618 |
| Tgene | HSP90AA1 | 102605587 | RPS6KA3 | 20252932 | ENST00000334701 | 0 | 22 | 68_327 | 23 | 741 | Domain | Note=Protein kinase 1;Ontology_term=ECO:0000255;evidence=ECO:0000255|PROSITE-ProRule:PRU00159 |
| Tgene | HSP90AA1 | 102605587 | RPS6KA3 | 20252932 | ENST00000334701 | 0 | 22 | 422_679 | 23 | 741 | Domain | Note=Protein kinase 2;Ontology_term=ECO:0000255;evidence=ECO:0000255|PROSITE-ProRule:PRU00159 |
Top |
Kinase Fusion Protein Structures |
 CIF files of the predicted kinase fusion proteins CIF files of the predicted kinase fusion proteins * Here we show the 3D structure of the fusion proteins using Mol*. AlphaFold produces a per-residue confidence score (pLDDT) between 0 and 100. Model confidence is shown from the pLDDT values per residue. pLDDT corresponds to the model’s prediction of its score on the local Distance Difference Test. It is a measure of local accuracy (from AlphfaFold website). To color code individual residues, we transformed individual PDB files into CIF format. |
| Kinase Fusion protein CIF link (fusion AA seq ID in KinaseFusionDB) | Henst | Tenst | Hgene | Hchr | Hbp | Tgene | Tchr | Tbp | AA seq | Len(AA seq) |
| PDB file >>>TKFP_421_HSP90AA1_RPS6KA3 | ENST00000334701 | ENST00000379565 | HSP90AA1 | chr14 | 102605587 | RPS6KA3 | chrX | 20252932 | MDEPMGEEEINPQTEEVSIKEIAITHHVKEGHEKADPSQFELLKVLGQGSFGKVFLVKKISGSDARQLYAMKVLKKATLKVRDRVRTKME RDILVEVNHPFIVKLHYAFQTEGKLYLILDFLRGGDLFTRLSKEVMFTEEDVKFYLAELALALDHLHSLGIIYRDLKPENILLDEEGHIK LTDFGLSKESIDHEKKAYSFCGTVEYMAPEVVNRRGHTQSADWWSFGVLMFEMLTGTLPFQGKDRKETMTMILKAKLGMPQFLSPEAQSL LRMLFKRNPANRLGAGPDGVEEIKRHSFFSTIDWNKLYRREIHPPFKPATGRPEDTFYFDPEFTAKTPKDSPGIPPSANAHQLFRGFSFV AITSDDESQAMQTVGVHSIVQQLHRNSIQFTDGYEVKEDIGVGSYSVCKRCIHKATNMEFAVKIIDKSKRDPTEEIEILLRYGQHPNIIT LKDVYDDGKYVYVVTELMKGGELLDKILRQKFFSEREASAVLFTITKTVEYLHAQGVVHRDLKPSNILYVDESGNPESIRICDFGFAKQL RAENGLLMTPCYTANFVAPEVLKRQGYDAACDIWSLGVLLYTMLTGYTPFANGPDDTPEEILARIGSGKFSLSGGYWNSVSDTAKDLVSK | 712_HSP90AA1_RPS6KA3 |
Top |
Comparison of Fusion Protein Isoforms |
 Superimpose the 3D Structures Among All Fusion Protein Isoforms Superimpose the 3D Structures Among All Fusion Protein Isoforms * Download the pdb file and open it from the molstar online viewer. |
 Comparison of the Secondary Structures of Fusion Protein Isoforms Comparison of the Secondary Structures of Fusion Protein Isoforms |
Top |
Comparison of Fusion Protein Sequences/Structures with Known Sequences/Structures from PDB |
Top |
pLDDT score distribution |
 pLDDT score distribution of the predicted fusion protein structures from AlphaFold2 pLDDT score distribution of the predicted fusion protein structures from AlphaFold2* AlphaFold produces a per-residue confidence score (pLDDT) between 0 and 100. * The blue color at the bottom marks the best active site residues. |
Top |
Potential Active Site Information |
 The potential binding sites of these fusion proteins were identified using SiteMap, a module of the Schrodinger suite. The potential binding sites of these fusion proteins were identified using SiteMap, a module of the Schrodinger suite. |
| Kinase Fusion AA seq ID in KinaseFusionDB | Site score | Size | Dscore | Volume | Exposure | Enclosure | Contact | Phobic | Philic | Balance | Don/Acc | Residues |
Top |
Ramachandran Plot of Kinase Fusion Protein Structure |
 Ramachandran plot of the torsional angles - phi (φ)and psi (ψ) - of the residues (amino acids) contained in this fusion protein peptide. Ramachandran plot of the torsional angles - phi (φ)and psi (ψ) - of the residues (amino acids) contained in this fusion protein peptide. |
Top |
Virtual Screening Results |
 Distribution of the average docking score across all approved kinase inhibitors. Distribution of the average docking score across all approved kinase inhibitors.Distribution of the number of occurrence across all approved kinase inhibitors. |
| 5'-kinase fusion protein case |
| 3'-kinase fusion protein case |
Top |
 Drug information from DrugBank of the top 20 interacting small molecules. Drug information from DrugBank of the top 20 interacting small molecules.* The detailed information of individual kinase inhibitors are available in the download page. |
| Fusion gene name info | Drug | Docking score | Glide g score | Glide energy |
Top |
Kinase-Substrate Information of HSP90AA1_RPS6KA3 |
 Phosphorylation target of the kinase Phosphorylation target of the kinase(phosphosite, 03-17-2024) |
| Kinase | Kinase UniProt Acc | Kinase species | Substrate | Substrate UniProt Acc | Substrate phosphorylated residues | Substrate phosphorylated sites (+/-7AA) | Domain |
| RPS6KA3 | P51812 | human | NFATC4 | Q14934 | S289 | PALsRRGsLGEEGsE | |
| RPS6KA3 | P51812 | human | WWC1 | Q8IX03 | S947 | CRLNRsDsDSSTLSK | |
| RPS6KA3 | P51812 | human | NFATC4 | Q14934 | S281 | SGTPSSAsPALsRRG | |
| RPS6KA3 | P51812 | human | TH | P07101-4 | S44 | RFIGRRQsLIEDARK | TOH_N |
| RPS6KA3 | P51812 | human | RIOK2 | Q9BVS4 | S483 | QYRTRtLsItssGsA | |
| RPS6KA3 | P51812 | human | EIF2AK2 | P19525 | T451 | kRtRSKGtLRyMsPE | Pkinase |
| RPS6KA3 | P51812 | human | YBX1 | P67809 | S102 | NPRKyLrsVGDGEtV | CSD |
| RPS6KA3 | P51812 | human | TINF2 | Q9BSI4 | S295 | FPFRNLGsPtQVISk | |
| RPS6KA3 | P51812 | human | H3C1 | P68431 | S10 | tkQtArkstGGkAPr | Histone |
| RPS6KA3 | P51812 | human | RRN3 | Q9NYV6 | S649 | PVLYMQPsPL_____ | |
| RPS6KA3 | P51812 | human | LCP1 | P13796 | S5 | ___MARGsVsDEEMM | |
| RPS6KA3 | P51812 | human | MYL12A | P19105 | S19 | KRPQRAtsNVFAMFD | |
| RPS6KA3 | P51812 | human | TBX21 | Q9UL17 | S503 | DSKRRRVsPYPsSGD | |
| RPS6KA3 | P51812 | human | TBX21 | Q9UL17 | S507 | RRVsPYPsSGDSsSP | |
| RPS6KA3 | P51812 | human | MAP3K5 | Q99683 | T1109 | DRKIIATtLSKLkLE | HisK-N-like |
| RPS6KA3 | P51812 | human | DAPK1 | P53355 | S289 | QALSRKAsAVNMEkF | |
| RPS6KA3 | P51812 | human | STAT3 | P40763 | S727 | NtIDLPMsPrTLDSL | |
| RPS6KA3 | P51812 | human | STMN1 | P16949 | S16 | kELEKrAsGQAFELI | Stathmin |
| RPS6KA3 | P51812 | human | EPHA2 | P29317 | S897 | RVsIRLPstsGsEGV | |
| RPS6KA3 | P51812 | human | BAD | Q92934 | S99 | PFrGrsRsAPPNLWA | Bcl-2_BAD |
| RPS6KA3 | P51812 | human | NFATC4 | Q14934 | S344 | QAVALPRsEEPASCN | |
| RPS6KA3 | P51812 | human | WWC1 | Q8IX03 | T929 | stIIRsKtFsPGPQS | |
| RPS6KA3 | P51812 | human | NFKBIA | P25963 | S32 | LLDDRHDsGLDsMkD | |
| RPS6KA3 | P51812 | human | GSK3A | P49840 | S21 | sGrARtssFAEPGGG | |
| RPS6KA3 | P51812 | human | PPP1R3A | Q16821 | S65 | SSGTRRVsFADSFGF | |
| RPS6KA3 | P51812 | human | NFATC4 | Q14934 | S676 | SNGRRKRsPTQSFRF | RHD_dimer |
| RPS6KA3 | P51812 | human | NFATC4 | Q14934 | S285 | SSAsPALsRRGsLGE | |
| RPS6KA3 | P51812 | human | SPRED2 | Q7Z698 | S168 | QPTRTIssPTSCEHR | |
| RPS6KA3 | P51812 | human | MAP3K5 | Q99683 | S83 | ATRGRGssVGGGSrR | |
| RPS6KA3 | P51812 | human | BAD | Q92934 | S75 | EIRsRHssyPAGtED | Bcl-2_BAD |
| RPS6KA3 | P51812 | human | CASP8 | Q14790 | T263 | SIRDRNGtHLDAGAL | Peptidase_C14 |
| RPS6KA3 | P51812 | human | CREB1 | P16220 | S119 | EILsRRPsyRkILND | pKID |
| RPS6KA3 | P51812 | human | GSK3B | P49841 | S9 | SGRPRttsFAEsCkP | |
| RPS6KA3 | P51812 | human | RANBP3 | Q9H6Z4 | S126 | VKRERtssLtQFPPs | |
| RPS6KA3 | P51812 | human | ESR1 | P03372 | S167 | GGRERLAsTNDkGSM | Oest_recep |
| RPS6KA3 | P51812 | human | H2AX | P16104 | S16 | kARAkAksRSsrAGL | Histone |
| RPS6KA3 | P51812 | human | RPS6 | P62753 | S236 | AKRRRLssLRAstsK | |
| RPS6KA3 | P51812 | human | VGLL1 | Q99990 | S84 | PNQWRYSsPWTKPQP | |
| RPS6KA3 | P51812 | human | RELA | Q04206 | S276 | sMQLRRPsDRELsEP | RHD_dimer |
| RPS6KA3 | P51812 | human | TINF2 | Q9BSI4 | S330 | ASTGKSKsPCQTLGG | |
| RPS6KA3 | P51812 | human | TH | P07101-2 | S67 | RFIGRRQsLIEDARK | TOH_N |
| RPS6KA3 | P51812 | human | MAP3K5 | Q99683 | T1326 | VNGADEDtISRFLAE | |
| RPS6KA3 | P51812 | human | TH | P07101 | S71 | RFIGRRQsLIEDARK | TOH_N |
| RPS6KA3 | P51812 | human | PPP1R3A | Q16821 | S46 | PQPSRRGsDssEDIY | |
| RPS6KA3 | P51812 | human | ATF4 | P18848 | S245 | TrGSPNRsLPsPGVL | |
| RPS6KA3 | P51812 | human | FOXN2 | P32314 | S369 | LGDsGYAsQPCAKIS | |
| RPS6KA3 | P51812 | human | FOXN2 | P32314 | S365 | EDDPLGDsGYAsQPC | |
| RPS6KA3 | P51812 | human | SPRED2 | Q7Z698 | S167 | EQPTRTIssPTSCEH | |
| RPS6KA3 | P51812 | human | H2BC3 | P33778 | S32 | DGKkRKRsRkEsysI | Histone |
| RPS6KA3 | P51812 | human | FGFR1 | P11362 | S789 | DTRSSTCsSGEDSVF | |
| RPS6KA3 | P51812 | human | RPS6 | P62753 | S235 | IAKRRRLssLRAsts | |
| RPS6KA3 | P51812 | human | SLC9A3 | P48764 | S663 | TMRKRLEsFKSTKLG | |
| RPS6KA3 | P51812 | human | TH | P07101-3 | S40 | RFIGRRQsLIEDARK | TOH_N |
| RPS6KA3 | P51812 | human | CIC | Q96RK0 | S173 | PGKRRtQsLsALPKE | |
| RPS6KA3 | P51812 | human | EIF2S1 | P05198 | S52 | MILLsELsRRRIRsI | S1 |
| RPS6KA3 | P51812 | human | KCNK3 | O14649 | S393 | GLMKRRssV______ |
 Biological Network Integration of This Kinase and Substrates Biological Network Integration of This Kinase and Substrates (GeneMANIA website) |
 Enriched GO biological processes of the phosphorylation target genes of the kinase Enriched GO biological processes of the phosphorylation target genes of the kinase |
| Kinase | GOID | GO term | P.adjust |
| RPS6KA3 | ID | Description | 0.00e+00 |
| RPS6KA3 | GO:0071248 | cellular response to metal ion | 2.28e-04 |
| RPS6KA3 | GO:0071241 | cellular response to inorganic substance | 2.28e-04 |
| RPS6KA3 | GO:0097191 | extrinsic apoptotic signaling pathway | 2.28e-04 |
| RPS6KA3 | GO:0010038 | response to metal ion | 2.56e-04 |
| RPS6KA3 | GO:0071287 | cellular response to manganese ion | 3.40e-04 |
| RPS6KA3 | GO:0034198 | cellular response to amino acid starvation | 1.04e-03 |
| RPS6KA3 | GO:1990928 | response to amino acid starvation | 1.04e-03 |
| RPS6KA3 | GO:0002064 | epithelial cell development | 1.04e-03 |
| RPS6KA3 | GO:0010042 | response to manganese ion | 1.15e-03 |
| RPS6KA3 | GO:0045862 | positive regulation of proteolysis | 1.15e-03 |
| RPS6KA3 | GO:0038034 | signal transduction in absence of ligand | 1.59e-03 |
| RPS6KA3 | GO:0097192 | extrinsic apoptotic signaling pathway in absence of ligand | 1.59e-03 |
| RPS6KA3 | GO:1902893 | regulation of miRNA transcription | 1.71e-03 |
| RPS6KA3 | GO:0061614 | miRNA transcription | 1.71e-03 |
| RPS6KA3 | GO:2001233 | regulation of apoptotic signaling pathway | 1.71e-03 |
| RPS6KA3 | GO:0003323 | type B pancreatic cell development | 1.71e-03 |
| RPS6KA3 | GO:0010950 | positive regulation of endopeptidase activity | 1.71e-03 |
| RPS6KA3 | GO:1902894 | negative regulation of miRNA transcription | 1.77e-03 |
| RPS6KA3 | GO:2000629 | negative regulation of miRNA metabolic process | 1.89e-03 |
| RPS6KA3 | GO:0010952 | positive regulation of peptidase activity | 2.20e-03 |
| RPS6KA3 | GO:0008625 | extrinsic apoptotic signaling pathway via death domain receptors | 2.33e-03 |
| RPS6KA3 | GO:2000628 | regulation of miRNA metabolic process | 2.33e-03 |
| RPS6KA3 | GO:0003309 | type B pancreatic cell differentiation | 2.43e-03 |
| RPS6KA3 | GO:0005979 | regulation of glycogen biosynthetic process | 2.47e-03 |
| RPS6KA3 | GO:0010962 | regulation of glucan biosynthetic process | 2.47e-03 |
| RPS6KA3 | GO:0140747 | regulation of ncRNA transcription | 2.57e-03 |
| RPS6KA3 | GO:0006109 | regulation of carbohydrate metabolic process | 2.64e-03 |
| RPS6KA3 | GO:0062197 | cellular response to chemical stress | 2.74e-03 |
| RPS6KA3 | GO:0097193 | intrinsic apoptotic signaling pathway | 2.74e-03 |
| RPS6KA3 | GO:0046777 | protein autophosphorylation | 3.02e-03 |
| RPS6KA3 | GO:0002068 | glandular epithelial cell development | 3.02e-03 |
| RPS6KA3 | GO:0071214 | cellular response to abiotic stimulus | 3.02e-03 |
| RPS6KA3 | GO:0104004 | cellular response to environmental stimulus | 3.02e-03 |
| RPS6KA3 | GO:0035883 | enteroendocrine cell differentiation | 3.02e-03 |
| RPS6KA3 | GO:0070306 | lens fiber cell differentiation | 3.02e-03 |
| RPS6KA3 | GO:0001819 | positive regulation of cytokine production | 3.02e-03 |
| RPS6KA3 | GO:0010586 | miRNA metabolic process | 3.02e-03 |
| RPS6KA3 | GO:0070873 | regulation of glycogen metabolic process | 3.02e-03 |
| RPS6KA3 | GO:0032885 | regulation of polysaccharide biosynthetic process | 3.12e-03 |
| RPS6KA3 | GO:0071496 | cellular response to external stimulus | 3.12e-03 |
| RPS6KA3 | GO:0043280 | positive regulation of cysteine-type endopeptidase activity involved in apoptotic process | 3.40e-03 |
| RPS6KA3 | GO:0032355 | response to estradiol | 3.56e-03 |
| RPS6KA3 | GO:0005978 | glycogen biosynthetic process | 4.15e-03 |
| RPS6KA3 | GO:0006984 | ER-nucleus signaling pathway | 4.15e-03 |
| RPS6KA3 | GO:0009250 | glucan biosynthetic process | 4.15e-03 |
| RPS6KA3 | GO:0031018 | endocrine pancreas development | 4.15e-03 |
| RPS6KA3 | GO:0032881 | regulation of polysaccharide metabolic process | 4.15e-03 |
| RPS6KA3 | GO:0042789 | mRNA transcription by RNA polymerase II | 4.15e-03 |
| RPS6KA3 | GO:0002286 | T cell activation involved in immune response | 4.15e-03 |
Top |
Related Drugs to HSP90AA1_RPS6KA3 |
 Drugs used for this fusion-positive patient. Drugs used for this fusion-positive patient. (Manual curation of PubMed, 04-30-2022 + MyCancerGenome) |
| Hgene | Tgene | Drug | Source | PMID |
 Distribution of the number of studies mentioning HSP90AA1-RPS6KA3 and kinase inhibitors the PubMed Abstract (04-01-2024) Distribution of the number of studies mentioning HSP90AA1-RPS6KA3 and kinase inhibitors the PubMed Abstract (04-01-2024) |
| Fusion gene - drug pair 1 | Fusion gene - drug pair 2 | PMID | Publication date | DOI | Study title |
Top |
Related Diseases to HSP90AA1_RPS6KA3 |
 Diseases that have this fusion gene. Diseases that have this fusion gene. (Manual curation of PubMed, 04-30-2022 + MyCancerGenome) |
| Hgene | Tgene | Disease | Source | PMID |
 Related diseases from the literature mentioned this fusion gene and drug. Related diseases from the literature mentioned this fusion gene and drug. (PubMed, 04-01-2024) |
| MeSH ID | MeSH term |
 Diseases associated with fusion partners. Diseases associated with fusion partners. (DisGeNet 4.0) |
| Partner | Gene | Disease ID | Disease name | # pubmeds | Source |
Top |
Clinical Trials of the Found Drugs/Small Molecules |
 Statistics of the Clinical Trials of the Found Kinase Inibitors from clinicaltrials.gov (06-17-2024) Statistics of the Clinical Trials of the Found Kinase Inibitors from clinicaltrials.gov (06-17-2024) |
 Clinical Trials from clinicaltrials.gov (06-17-2024) Clinical Trials from clinicaltrials.gov (06-17-2024) |
| Fusion Gene | Kinase Inhibitor | NCT ID | Study Status | Phases | Disease | # Enrolment | Date |

