| UTHEALTH HOME ABOUT SBMI A-Z WEBMAIL INSIDE THE UNIVERSITY |

|
|||||||
|
Kinase Fusion Gene:LMNA_RAF1 |
Kinase Fusion Protein Summary |
 Kinase Fusion gene summary Kinase Fusion gene summary |
| Kinase Fusion partner gene information | Kinase Fusion gene name: LMNA_RAF1 | KinaseFusionDB ID: KFG3176 | FusionGDB2.0 ID: KFG3176 | Hgene | Tgene | Gene symbol | LMNA | RAF1 | Gene ID | 4000 | 6037 | |
| Gene name | lamin A/C | |||||||||||
| Synonyms | CDCD1|CDDC|CMD1A|CMT2B1|EMD2|FPL|FPLD|FPLD2|HGPS|IDC|LDP1|LFP|LGMD1B|LMN1|LMNC|LMNL1|MADA|PRO1 | |||||||||||
| Cytomap | 1q22 | |||||||||||
| Type of gene | protein-coding | |||||||||||
| Description | lamin70 kDa laminepididymis secretory sperm binding proteinlamin A/C-like 1lamin Cmandibuloacral dysplasia type Aprelamin-A/Cprogerinrenal carcinoma antigen NY-REN-32 | |||||||||||
| Modification date | 20240411 | |||||||||||
| UniProtAcc | P02545 | P04049 | ||||||||||
| Ensembl transtripts involved in fusion gene | ENST ids | ENST00000496738, ENST00000361308, ENST00000368297, ENST00000368301, ENST00000392353, ENST00000368299, ENST00000368300, ENST00000448611, ENST00000473598, ENST00000347559, | ENST00000251849, ENST00000442415, ENST00000534997, ENST00000542177, | |||||||||
| Context (manual curation of fusion genes in KinaseFusionDB) | PubMed: LMNA [Title/Abstract] AND RAF1 [Title/Abstract] AND fusion [Title/Abstract] | |||||||||||
| Most frequent breakpoint (based on all fusion genes of FusionGDB 2.0) | LMNA(156107534)-RAF1(12641914), # samples:2 | |||||||||||
 Gene ontology of each fusion partner gene with evidence of Inferred from Direct Assay (IDA) from Entrez Gene ontology of each fusion partner gene with evidence of Inferred from Direct Assay (IDA) from Entrez |
| Partner | Gene | GO ID | GO term | PubMed ID |
| Hgene | LMNA | GO:0006998 | nuclear envelope organization | 2188730|24741066|37788673|37832547 |
| Hgene | LMNA | GO:0090398 | cellular senescence | 20458013 |
| Hgene | LMNA | GO:1990683 | DNA double-strand break attachment to nuclear envelope | 31548606 |
 Kinase Fusion gene breakpoints across LMNA (5'-gene) Kinase Fusion gene breakpoints across LMNA (5'-gene)* Click on the image to open the UCSC genome browser with custom track showing this image in a new window. |
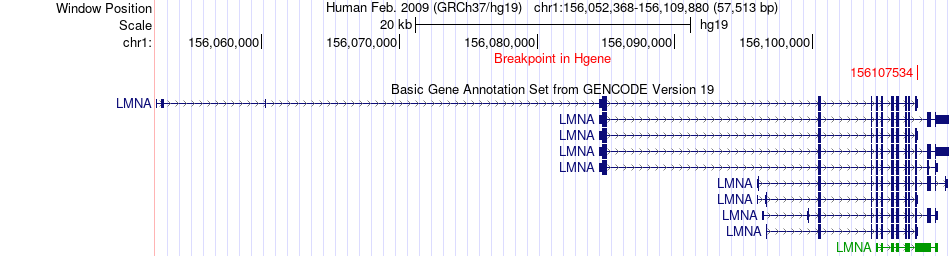 |
 Kinase Fusion gene breakpoints across RAF1 (3'-gene) Kinase Fusion gene breakpoints across RAF1 (3'-gene)* Click on the image to open the UCSC genome browser with custom track showing this image in a new window. |
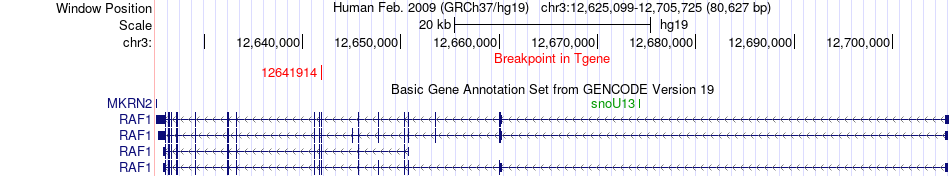 |
Top |
Kinase Fusion Gene Sample Information |
 Kinase Fusion gene information. Kinase Fusion gene information. |
 Kinase Fusion gene information from four resources (ChiTars 5.0, ChimerDB 4.0, COSMIC, and CCLE) Kinase Fusion gene information from four resources (ChiTars 5.0, ChimerDB 4.0, COSMIC, and CCLE)* All genome coordinats were lifted-over on hg19. * Click on the break point to see the gene structure around the break point region using the UCSC Genome Browser. |
| Source | Sample | Hgene | Hchr | Hbp | Tgene | Tchr | Tbp |
| ChimerDB4 | TCGA-EB-A5SF-01A | LMNA | chr1 | 156107534 | RAF1 | chr3 | 12641914 |
Top |
Kinase Fusion ORF Analysis |
 Kinase Fusion information from ORFfinder translation from full-length transcript sequence from KinaseFusionDB. Kinase Fusion information from ORFfinder translation from full-length transcript sequence from KinaseFusionDB. |
| Henst | Tenst | Hgene | Hchr | Hbp | Tgene | Tchr | Tbp | Seq length (transcript) | Seq length (amino acids) |
| ENST00000368300 | ENST00000251849 | LMNA | chr1 | 156107534 | RAF1 | chr3 | 12641914 | 3936 | 979 |
Top |
Kinase Fusion Amino Acid Sequences |
 For individual full-length fusion transcript sequence from KinaseFusionDB, we ran ORFfinder and chose the longest ORF among the all predicted ones. For individual full-length fusion transcript sequence from KinaseFusionDB, we ran ORFfinder and chose the longest ORF among the all predicted ones. |
| >Henst_Tenst_Hgene_Hchr_Hbp_Tgene_Tchr_Tbp_length(fusion AA)_AAseq >ENST00000368300_ENST00000251849_LMNA_chr1_156107534_RAF1_chr3_12641914_length(amino acids)=979 MPGASREPAGRRTPTPSSLCPSTRAPRPFRDPCPAGSAANLPAMETPSQRRATRSGAQASSTPLSPTRITRLQEKEDLQELNDRLAVYID RVRSLETENAGLRLRITESEEVVSREVSGIKAAYEAELGDARKTLDSVAKERARLQLELSKVREEFKELKARNTKKEGDLIAAQARLKDL EALLNSKEAALSTALSEKRTLEGELHDLRGQVAKLEAALGEAKKQLQDEMLRRVDAENRLQTMKEELDFQKNIYSEELRETKRRHETRLV EIDNGKQREFESRLADALQELRAQHEDQVEQYKKELEKTYSAKLDNARQSAERNSNLVGAAHEELQQSRIRIDSLSAQLSQLQKQLAAKE AKLRDLEDSLARERDTSRRLLAEKEREMAEMRARMQQQLDEYQELLDIKLALDMEIHAYRKLLEGEEERLRLSPSPTSQRSRGRASSHSS QTQGGGSVTKKRKLESTESRSSFSQHARTSGRVAVEEVDEEGKFVRLRNKSNEDQSMGNWQIKRQNGDDPLLTYRFPPKFTLKAGQVVTI WAAGAGATHSPPTDLVWKAQNTWGCGNSLRTALINSTGEEVAMRKLVRSVTVVEDDEDEDGDDLLHHHHDAIRSHSESASPSALSSSPNN LSPTGWSQPKTPVPAQRERAPVSGTQEKNKIRPRGQRDSSYYWEIEASEVMLSTRIGSGSFGTVYKGKWHGDVAVKILKVVDPTPEQFQA FRNEVAVLRKTRHVNILLFMGYMTKDNLAIVTQWCEGSSLYKHLHVQETKFQMFQLIDIARQTAQGMDYLHAKNIIHRDMKSNNIFLHEG LTVKIGDFGLATVKSRWSGSQQVEQPTGSVLWMAPEVIRMQDNNPFSFQSDVYSYGIVLYELMTGELPYSHINNRDQIIFMVGRGYASPD -------------------------------------------------------------- |
Multiple Sequence Alignment of All Fusion Protein Isoforms |
Top |
Kinase Fusion Protein Functional Features |
 Four levels of functional features of fusion genes Four levels of functional features of fusion genesGo to FGviewer search page for the most frequent breakpoint (https://ccsmweb.uth.edu/FGviewer/chr1:156107534/chr3:12641914) - FGviewer provides the online visualization of the retention search of the protein functional features across DNA, RNA, protein, and pathological levels. - How to search 1. Put your fusion gene symbol. 2. Press the tab key until there will be shown the breakpoint information filled. 4. Go down and press 'Search' tab twice. 4. Go down to have the hyperlink of the search result. 5. Click the hyperlink. 6. See the FGviewer result for your fusion gene. |
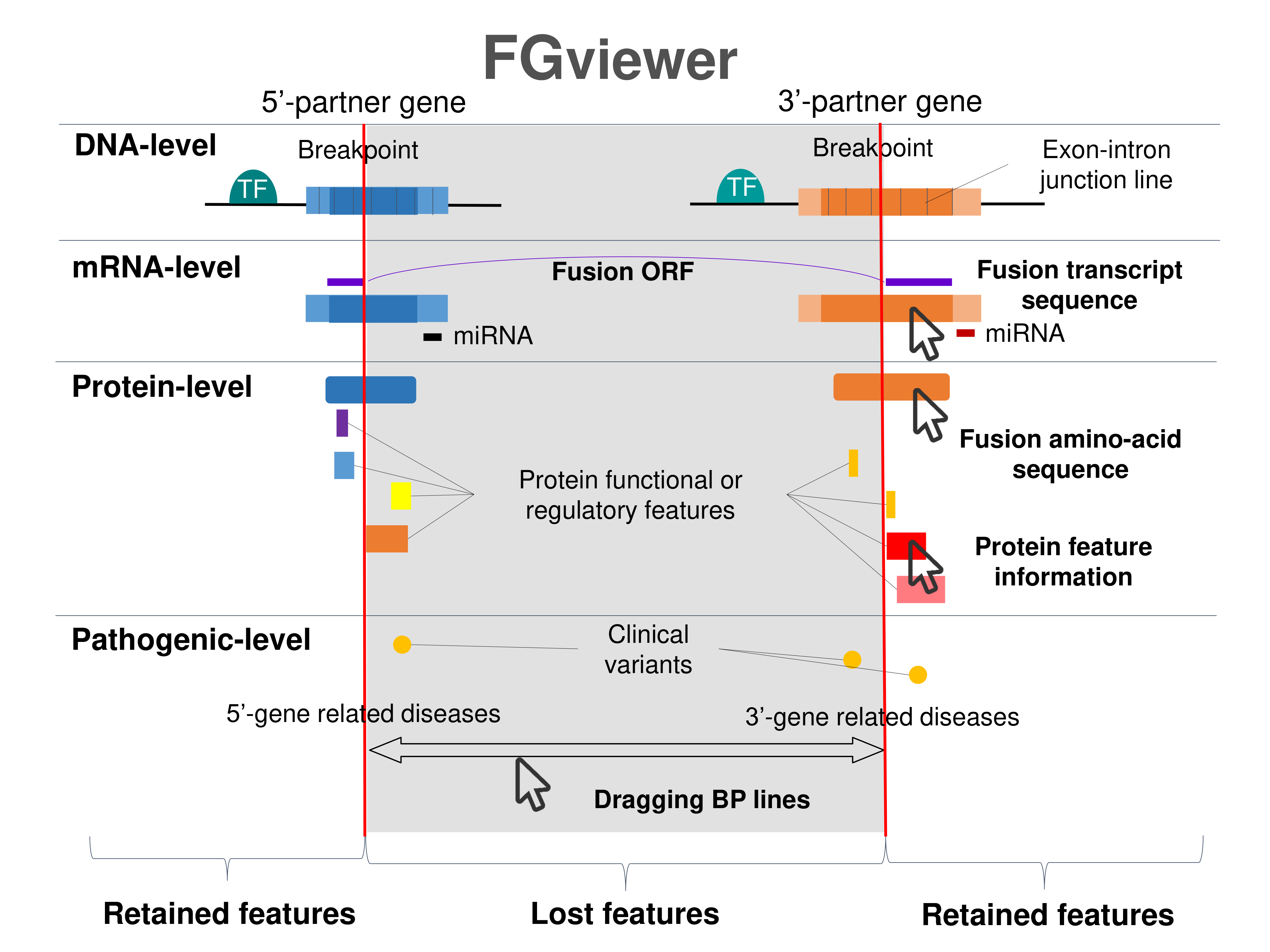 |
 Main function of each fusion partner protein. (from UniProt) Main function of each fusion partner protein. (from UniProt) |
| Hgene | Tgene |
| LMNA | RAF1 |
| FUNCTION: Lamins are components of the nuclear lamina, a fibrous layer on the nucleoplasmic side of the inner nuclear membrane, which is thought to provide a framework for the nuclear envelope and may also interact with chromatin (PubMed:10080180, PubMed:10580070, PubMed:10587585, PubMed:10814726, PubMed:11799477, PubMed:12075506, PubMed:12927431, PubMed:15317753, PubMed:18551513, PubMed:18611980, PubMed:22431096, PubMed:23666920, PubMed:31548606). Lamin A and C are present in equal amounts in the lamina of mammals (PubMed:10080180, PubMed:10580070, PubMed:10587585, PubMed:10814726, PubMed:11799477, PubMed:12075506, PubMed:12927431, PubMed:15317753, PubMed:18551513, PubMed:18611980, PubMed:22431096, PubMed:23666920, PubMed:31548606). Recruited by DNA repair proteins XRCC4 and IFFO1 to the DNA double-strand breaks (DSBs) to prevent chromosome translocation by immobilizing broken DNA ends (PubMed:31548606). Plays an important role in nuclear assembly, chromatin organization, nuclear membrane and telomere dynamics. Required for normal development of peripheral nervous system and skeletal muscle and for muscle satellite cell proliferation (PubMed:10080180, PubMed:10814726, PubMed:11799477, PubMed:18551513, PubMed:22431096). Required for osteoblastogenesis and bone formation (PubMed:12075506, PubMed:15317753, PubMed:18611980). Also prevents fat infiltration of muscle and bone marrow, helping to maintain the volume and strength of skeletal muscle and bone (PubMed:10587585). Required for cardiac homeostasis (PubMed:10580070, PubMed:12927431, PubMed:23666920, PubMed:18611980). {ECO:0000269|PubMed:10080180, ECO:0000269|PubMed:10580070, ECO:0000269|PubMed:10587585, ECO:0000269|PubMed:10814726, ECO:0000269|PubMed:11799477, ECO:0000269|PubMed:12075506, ECO:0000269|PubMed:12927431, ECO:0000269|PubMed:15317753, ECO:0000269|PubMed:18551513, ECO:0000269|PubMed:18611980, ECO:0000269|PubMed:22431096, ECO:0000269|PubMed:23666920, ECO:0000269|PubMed:31548606}.; FUNCTION: Prelamin-A/C can accelerate smooth muscle cell senescence. It acts to disrupt mitosis and induce DNA damage in vascular smooth muscle cells (VSMCs), leading to mitotic failure, genomic instability, and premature senescence. {ECO:0000269|PubMed:20458013}. | FUNCTION: Serine/threonine-protein kinase that acts as a regulatory link between the membrane-associated Ras GTPases and the MAPK/ERK cascade, and this critical regulatory link functions as a switch determining cell fate decisions including proliferation, differentiation, apoptosis, survival and oncogenic transformation. RAF1 activation initiates a mitogen-activated protein kinase (MAPK) cascade that comprises a sequential phosphorylation of the dual-specific MAPK kinases (MAP2K1/MEK1 and MAP2K2/MEK2) and the extracellular signal-regulated kinases (MAPK3/ERK1 and MAPK1/ERK2). The phosphorylated form of RAF1 (on residues Ser-338 and Ser-339, by PAK1) phosphorylates BAD/Bcl2-antagonist of cell death at 'Ser-75'. Phosphorylates adenylyl cyclases: ADCY2, ADCY5 and ADCY6, resulting in their activation. Phosphorylates PPP1R12A resulting in inhibition of the phosphatase activity. Phosphorylates TNNT2/cardiac muscle troponin T. Can promote NF-kB activation and inhibit signal transducers involved in motility (ROCK2), apoptosis (MAP3K5/ASK1 and STK3/MST2), proliferation and angiogenesis (RB1). Can protect cells from apoptosis also by translocating to the mitochondria where it binds BCL2 and displaces BAD/Bcl2-antagonist of cell death. Regulates Rho signaling and migration, and is required for normal wound healing. Plays a role in the oncogenic transformation of epithelial cells via repression of the TJ protein, occludin (OCLN) by inducing the up-regulation of a transcriptional repressor SNAI2/SLUG, which induces down-regulation of OCLN. Restricts caspase activation in response to selected stimuli, notably Fas stimulation, pathogen-mediated macrophage apoptosis, and erythroid differentiation. {ECO:0000269|PubMed:11427728, ECO:0000269|PubMed:11719507, ECO:0000269|PubMed:15385642, ECO:0000269|PubMed:15618521, ECO:0000269|PubMed:15849194, ECO:0000269|PubMed:16892053, ECO:0000269|PubMed:16924233, ECO:0000269|PubMed:9360956}. |
 Retention analysis result of each fusion partner protein across 39 protein features of UniProt such as six molecule processing features, 13 region features, four site features, six amino acid modification features, two natural variation features, five experimental info features, and 3 secondary structure features. Here, because of limited space for viewing, we only show the protein feature retention information belong to the 13 regional features. All retention annotation result can be downloaded at * Minus value of BPloci means that the break pointn is located before the CDS. Retention analysis result of each fusion partner protein across 39 protein features of UniProt such as six molecule processing features, 13 region features, four site features, six amino acid modification features, two natural variation features, five experimental info features, and 3 secondary structure features. Here, because of limited space for viewing, we only show the protein feature retention information belong to the 13 regional features. All retention annotation result can be downloaded at * Minus value of BPloci means that the break pointn is located before the CDS. |
 - Retained domain in the 5'-partner of fusion protein (protein functional feature from UniProt). - Retained domain in the 5'-partner of fusion protein (protein functional feature from UniProt). |
| Partner | Hgeneene | Hbp | Tgeneene | Tbp | ENST | BPexon | TotalExon | Protein feature loci | BPloci | TotalLen | Feature | Note |
 - Retained domain in the 3'-partner of fusion protein (protein functional feature from UniProt). - Retained domain in the 3'-partner of fusion protein (protein functional feature from UniProt). |
| Partner | Hgeneene | Hbp | Tgeneene | Tbp | ENST | BPexon | TotalExon | Protein feature loci | BPloci | TotalLen | Feature | Note |
| Tgene | LMNA | 156107534 | RAF1 | 12641914 | ENST00000368300 | 6 | 17 | 349_609 | 278 | 649 | Domain | Note=Protein kinase;Ontology_term=ECO:0000255;evidence=ECO:0000255|PROSITE-ProRule:PRU00159 |
| Tgene | LMNA | 156107534 | RAF1 | 12641914 | ENST00000368300 | 7 | 18 | 349_609 | 298 | 669 | Domain | Note=Protein kinase;Ontology_term=ECO:0000255;evidence=ECO:0000255|PROSITE-ProRule:PRU00159 |
Top |
Kinase Fusion Protein Structures |
 CIF files of the predicted kinase fusion proteins CIF files of the predicted kinase fusion proteins * Here we show the 3D structure of the fusion proteins using Mol*. AlphaFold produces a per-residue confidence score (pLDDT) between 0 and 100. Model confidence is shown from the pLDDT values per residue. pLDDT corresponds to the model’s prediction of its score on the local Distance Difference Test. It is a measure of local accuracy (from AlphfaFold website). To color code individual residues, we transformed individual PDB files into CIF format. |
| Kinase Fusion protein CIF link (fusion AA seq ID in KinaseFusionDB) | Henst | Tenst | Hgene | Hchr | Hbp | Tgene | Tchr | Tbp | AA seq | Len(AA seq) |
| PDB file >>>284_LMNA_RAF1 | ENST00000368300 | ENST00000251849 | LMNA | chr1 | 156107534 | RAF1 | chr3 | 12641914 | MPGASREPAGRRTPTPSSLCPSTRAPRPFRDPCPAGSAANLPAMETPSQRRATRSGAQASSTPLSPTRITRLQEKEDLQELNDRLAVYID RVRSLETENAGLRLRITESEEVVSREVSGIKAAYEAELGDARKTLDSVAKERARLQLELSKVREEFKELKARNTKKEGDLIAAQARLKDL EALLNSKEAALSTALSEKRTLEGELHDLRGQVAKLEAALGEAKKQLQDEMLRRVDAENRLQTMKEELDFQKNIYSEELRETKRRHETRLV EIDNGKQREFESRLADALQELRAQHEDQVEQYKKELEKTYSAKLDNARQSAERNSNLVGAAHEELQQSRIRIDSLSAQLSQLQKQLAAKE AKLRDLEDSLARERDTSRRLLAEKEREMAEMRARMQQQLDEYQELLDIKLALDMEIHAYRKLLEGEEERLRLSPSPTSQRSRGRASSHSS QTQGGGSVTKKRKLESTESRSSFSQHARTSGRVAVEEVDEEGKFVRLRNKSNEDQSMGNWQIKRQNGDDPLLTYRFPPKFTLKAGQVVTI WAAGAGATHSPPTDLVWKAQNTWGCGNSLRTALINSTGEEVAMRKLVRSVTVVEDDEDEDGDDLLHHHHDAIRSHSESASPSALSSSPNN LSPTGWSQPKTPVPAQRERAPVSGTQEKNKIRPRGQRDSSYYWEIEASEVMLSTRIGSGSFGTVYKGKWHGDVAVKILKVVDPTPEQFQA FRNEVAVLRKTRHVNILLFMGYMTKDNLAIVTQWCEGSSLYKHLHVQETKFQMFQLIDIARQTAQGMDYLHAKNIIHRDMKSNNIFLHEG LTVKIGDFGLATVKSRWSGSQQVEQPTGSVLWMAPEVIRMQDNNPFSFQSDVYSYGIVLYELMTGELPYSHINNRDQIIFMVGRGYASPD | 979 |
| 3D view using mol* of 284_LMNA_RAF1 | ||||||||||
| PDB file >>>TKFP_498_LMNA_RAF1 | ENST00000368300 | ENST00000251849 | LMNA | chr1 | 156107534 | RAF1 | chr3 | 12641914 | MPGASREPAGRRTPTPSSLCPSTRAPRPFRDPCPAGSAANLPAMETPSQRRATRSGAQASSTPLSPTRITRLQEKEDLQELNDRLAVYID RVRSLETENAGLRLRITESEEVVSREVSGIKAAYEAELGDARKTLDSVAKERARLQLELSKVREEFKELKARNTKKEGDLIAAQARLKDL EALLNSKEAALSTALSEKRTLEGELHDLRGQVAKLEAALGEAKKQLQDEMLRRVDAENRLQTMKEELDFQKNIYSEELRETKRRHETRLV EIDNGKQREFESRLADALQELRAQHEDQVEQYKKELEKTYSAKLDNARQSAERNSNLVGAAHEELQQSRIRIDSLSAQLSQLQKQLAAKE AKLRDLEDSLARERDTSRRLLAEKEREMAEMRARMQQQLDEYQELLDIKLALDMEIHAYRKLLEGEEERLRLSPSPTSQRSRGRASSHSS QTQGGGSVTKKRKLESTESRSSFSQHARTSGRVAVEEVDEEGKFVRLRNKSNEDQSMGNWQIKRQNGDDPLLTYRFPPKFTLKAGQVVTI WAAGAGATHSPPTDLVWKAQNTWGCGNSLRTALINSTGEEVAMRKLVRSVTVVEDDEDEDGDDLLHHHHDAIRSHSESASPSALSSSPNN LSPTGWSQPKTPVPAQRERAPVSGTQEKNKIRPRGQRDSSYYWEIEASEVMLSTRIGSGSFGTVYKGKWHGDVAVKILKVVDPTPEQFQA FRNEVAVLRKTRHVNILLFMGYMTKDNLAIVTQWCEGSSLYKHLHVQETKFQMFQLIDIARQTAQGMDYLHAKNIIHRDMKSNNIFLHEG LTVKIGDFGLATVKSRWSGSQQVEQPTGSVLWMAPEVIRMQDNNPFSFQSDVYSYGIVLYELMTGELPYSHINNRDQIIFMVGRGYASPD | 979_LMNA_RAF1 |
Top |
Comparison of Fusion Protein Isoforms |
 Superimpose the 3D Structures Among All Fusion Protein Isoforms Superimpose the 3D Structures Among All Fusion Protein Isoforms * Download the pdb file and open it from the molstar online viewer. |
 Comparison of the Secondary Structures of Fusion Protein Isoforms Comparison of the Secondary Structures of Fusion Protein Isoforms |
Top |
Comparison of Fusion Protein Sequences/Structures with Known Sequences/Structures from PDB |
Top |
pLDDT score distribution |
 pLDDT score distribution of the predicted fusion protein structures from AlphaFold2 pLDDT score distribution of the predicted fusion protein structures from AlphaFold2* AlphaFold produces a per-residue confidence score (pLDDT) between 0 and 100. * The blue color at the bottom marks the best active site residues. |
Top |
Potential Active Site Information |
 The potential binding sites of these fusion proteins were identified using SiteMap, a module of the Schrodinger suite. The potential binding sites of these fusion proteins were identified using SiteMap, a module of the Schrodinger suite. |
| Kinase Fusion AA seq ID in KinaseFusionDB | Site score | Size | Dscore | Volume | Exposure | Enclosure | Contact | Phobic | Philic | Balance | Don/Acc | Residues |
Top |
Ramachandran Plot of Kinase Fusion Protein Structure |
 Ramachandran plot of the torsional angles - phi (φ)and psi (ψ) - of the residues (amino acids) contained in this fusion protein peptide. Ramachandran plot of the torsional angles - phi (φ)and psi (ψ) - of the residues (amino acids) contained in this fusion protein peptide. |
| 284_LMNA_RAF1_ramachandran.png |
 |
Top |
Virtual Screening Results |
 Distribution of the average docking score across all approved kinase inhibitors. Distribution of the average docking score across all approved kinase inhibitors.Distribution of the number of occurrence across all approved kinase inhibitors. |
| 5'-kinase fusion protein case |
| 3'-kinase fusion protein case |
Top |
 Drug information from DrugBank of the top 20 interacting small molecules. Drug information from DrugBank of the top 20 interacting small molecules.* The detailed information of individual kinase inhibitors are available in the download page. |
| Fusion gene name info | Drug | Docking score | Glide g score | Glide energy |
Top |
Kinase-Substrate Information of LMNA_RAF1 |
 Phosphorylation target of the kinase Phosphorylation target of the kinase(phosphosite, 03-17-2024) |
| Kinase | Kinase UniProt Acc | Kinase species | Substrate | Substrate UniProt Acc | Substrate phosphorylated residues | Substrate phosphorylated sites (+/-7AA) | Domain |
| RAF1 | P04049 | human | RAF1 | P04049 | S621 | PKINRsAsEPsLHRA | |
| RAF1 | P04049 | human | KLF10 | Q13118 | T93 | TIPAFCLtPPYsPSD | |
| RAF1 | P04049 | human | BAD | Q92934 | S75 | EIRsRHssyPAGtED | Bcl-2_BAD |
| RAF1 | P04049 | human | BAD | Q92934 | S118 | GRELRRMsDEFVDsF | Bcl-2_BAD |
| RAF1 | P04049 | human | MAP2K1 | Q02750 | S222 | LIDsMANsFVGtRSY | Pkinase |
| RAF1 | P04049 | human | RAF1 | P04049 | S359 | StRIGsGsFGtVYkG | PK_Tyr_Ser-Thr |
| RAF1 | P04049 | human | EEF1A2 | Q05639 | S21 | GHVDSGkstttGHLI | GTP_EFTU |
| RAF1 | P04049 | human | MAP2K1 | Q02750 | S218 | VsGQLIDsMANsFVG | Pkinase |
| RAF1 | P04049 | human | RAF1 | P04049 | S259 | sQRQRststPNVHMV | |
| RAF1 | P04049 | human | MYC | P01106 | T23 | MPLNVsFtNRNyDLD | Myc_N |
| RAF1 | P04049 | human | RAF1 | P04049 | S338 | RPRGQRDssyyWEIE | |
| RAF1 | P04049 | human | BAD | Q92934 | S99 | PFrGrsRsAPPNLWA | Bcl-2_BAD |
| RAF1 | P04049 | human | PPP1R12A | O14974 | T696 | ARQsRRstQGVtLtD |
 Biological Network Integration of This Kinase and Substrates Biological Network Integration of This Kinase and Substrates (GeneMANIA website) |
 Enriched GO biological processes of the phosphorylation target genes of the kinase Enriched GO biological processes of the phosphorylation target genes of the kinase |
| Kinase | GOID | GO term | P.adjust |
| RAF1 | ID | Description | 0.00e+00 |
| RAF1 | GO:0044342 | type B pancreatic cell proliferation | 4.58e-05 |
| RAF1 | GO:0035019 | somatic stem cell population maintenance | 3.64e-04 |
| RAF1 | GO:0051347 | positive regulation of transferase activity | 1.14e-03 |
| RAF1 | GO:0035270 | endocrine system development | 1.22e-03 |
| RAF1 | GO:0050673 | epithelial cell proliferation | 1.22e-03 |
| RAF1 | GO:0043281 | regulation of cysteine-type endopeptidase activity involved in apoptotic process | 1.62e-03 |
| RAF1 | GO:0019827 | stem cell population maintenance | 1.83e-03 |
| RAF1 | GO:0098727 | maintenance of cell number | 1.83e-03 |
| RAF1 | GO:2000116 | regulation of cysteine-type endopeptidase activity | 1.84e-03 |
| RAF1 | GO:0030878 | thyroid gland development | 2.45e-03 |
| RAF1 | GO:0052548 | regulation of endopeptidase activity | 4.55e-03 |
| RAF1 | GO:0048538 | thymus development | 4.55e-03 |
| RAF1 | GO:0052547 | regulation of peptidase activity | 4.55e-03 |
| RAF1 | GO:0048009 | insulin-like growth factor receptor signaling pathway | 4.55e-03 |
| RAF1 | GO:0060324 | face development | 4.55e-03 |
| RAF1 | GO:1902105 | regulation of leukocyte differentiation | 4.55e-03 |
| RAF1 | GO:0033674 | positive regulation of kinase activity | 4.95e-03 |
| RAF1 | GO:0035773 | insulin secretion involved in cellular response to glucose stimulus | 5.56e-03 |
| RAF1 | GO:2001244 | positive regulation of intrinsic apoptotic signaling pathway | 5.56e-03 |
| RAF1 | GO:2001233 | regulation of apoptotic signaling pathway | 6.87e-03 |
| RAF1 | GO:1903706 | regulation of hemopoiesis | 7.50e-03 |
| RAF1 | GO:0010720 | positive regulation of cell development | 8.29e-03 |
| RAF1 | GO:0008625 | extrinsic apoptotic signaling pathway via death domain receptors | 8.29e-03 |
| RAF1 | GO:0048534 | hematopoietic or lymphoid organ development | 1.03e-02 |
| RAF1 | GO:0043280 | positive regulation of cysteine-type endopeptidase activity involved in apoptotic process | 1.27e-02 |
| RAF1 | GO:0002761 | regulation of myeloid leukocyte differentiation | 1.52e-02 |
| RAF1 | GO:2001056 | positive regulation of cysteine-type endopeptidase activity | 1.58e-02 |
| RAF1 | GO:0071333 | cellular response to glucose stimulus | 1.68e-02 |
| RAF1 | GO:0071331 | cellular response to hexose stimulus | 1.68e-02 |
| RAF1 | GO:0071326 | cellular response to monosaccharide stimulus | 1.68e-02 |
| RAF1 | GO:2001235 | positive regulation of apoptotic signaling pathway | 1.73e-02 |
| RAF1 | GO:0071456 | cellular response to hypoxia | 1.73e-02 |
| RAF1 | GO:0071322 | cellular response to carbohydrate stimulus | 1.77e-02 |
| RAF1 | GO:0010950 | positive regulation of endopeptidase activity | 1.78e-02 |
| RAF1 | GO:0001678 | intracellular glucose homeostasis | 1.78e-02 |
| RAF1 | GO:0036294 | cellular response to decreased oxygen levels | 1.78e-02 |
| RAF1 | GO:0010952 | positive regulation of peptidase activity | 2.00e-02 |
| RAF1 | GO:0071453 | cellular response to oxygen levels | 2.03e-02 |
| RAF1 | GO:1902107 | positive regulation of leukocyte differentiation | 2.10e-02 |
| RAF1 | GO:1903708 | positive regulation of hemopoiesis | 2.10e-02 |
| RAF1 | GO:0032872 | regulation of stress-activated MAPK cascade | 2.10e-02 |
| RAF1 | GO:0070302 | regulation of stress-activated protein kinase signaling cascade | 2.10e-02 |
| RAF1 | GO:2001242 | regulation of intrinsic apoptotic signaling pathway | 2.10e-02 |
| RAF1 | GO:0071466 | cellular response to xenobiotic stimulus | 2.10e-02 |
| RAF1 | GO:0009749 | response to glucose | 2.10e-02 |
| RAF1 | GO:0009746 | response to hexose | 2.10e-02 |
| RAF1 | GO:0002520 | immune system development | 2.10e-02 |
| RAF1 | GO:0030073 | insulin secretion | 2.10e-02 |
| RAF1 | GO:0034284 | response to monosaccharide | 2.20e-02 |
Top |
Related Drugs to LMNA_RAF1 |
 Drugs used for this fusion-positive patient. Drugs used for this fusion-positive patient. (Manual curation of PubMed, 04-30-2022 + MyCancerGenome) |
| Hgene | Tgene | Drug | Source | PMID |
 Distribution of the number of studies mentioning LMNA-RAF1 and kinase inhibitors the PubMed Abstract (04-01-2024) Distribution of the number of studies mentioning LMNA-RAF1 and kinase inhibitors the PubMed Abstract (04-01-2024) |
| Fusion gene - drug pair 1 | Fusion gene - drug pair 2 | PMID | Publication date | DOI | Study title |
Top |
Related Diseases to LMNA_RAF1 |
 Diseases that have this fusion gene. Diseases that have this fusion gene. (Manual curation of PubMed, 04-30-2022 + MyCancerGenome) |
| Hgene | Tgene | Disease | Source | PMID |
 Related diseases from the literature mentioned this fusion gene and drug. Related diseases from the literature mentioned this fusion gene and drug. (PubMed, 04-01-2024) |
| MeSH ID | MeSH term |
 Diseases associated with fusion partners. Diseases associated with fusion partners. (DisGeNet 4.0) |
| Partner | Gene | Disease ID | Disease name | # pubmeds | Source |
| Tgene | RAF1 | C0028326 | Noonan Syndrome | 10 | CLINGEN;CTD_human;GENOMICS_ENGLAND;ORPHANET |
| Tgene | RAF1 | C0175704 | LEOPARD Syndrome | 7 | CLINGEN;CTD_human;GENOMICS_ENGLAND |
| Tgene | RAF1 | C1969057 | Noonan Syndrome 5 | 4 | CTD_human;GENOMICS_ENGLAND;UNIPROT |
| Tgene | RAF1 | C1969056 | LEOPARD SYNDROME 2 | 3 | CTD_human;GENOMICS_ENGLAND;UNIPROT |
| Tgene | RAF1 | C0007194 | Hypertrophic Cardiomyopathy | 2 | CTD_human |
| Tgene | RAF1 | C0041409 | Turner Syndrome, Male | 2 | CTD_human |
| Tgene | RAF1 | C1519086 | Pilomyxoid astrocytoma | 2 | ORPHANET |
| Tgene | RAF1 | C1527404 | Female Pseudo-Turner Syndrome | 2 | CTD_human |
| Tgene | RAF1 | C4551472 | Hypertrophic obstructive cardiomyopathy | 2 | CTD_human |
| Tgene | RAF1 | C4551602 | Noonan Syndrome 1 | 2 | CTD_human |
Top |
Clinical Trials of the Found Drugs/Small Molecules |
 Statistics of the Clinical Trials of the Found Kinase Inibitors from clinicaltrials.gov (06-17-2024) Statistics of the Clinical Trials of the Found Kinase Inibitors from clinicaltrials.gov (06-17-2024) |
 Clinical Trials from clinicaltrials.gov (06-17-2024) Clinical Trials from clinicaltrials.gov (06-17-2024) |
| Fusion Gene | Kinase Inhibitor | NCT ID | Study Status | Phases | Disease | # Enrolment | Date |

