| UTHEALTH HOME ABOUT SBMI A-Z WEBMAIL INSIDE THE UNIVERSITY |

|
|||||||
|
Kinase Fusion Gene:ANP32B_PRKG1 |
Kinase Fusion Protein Summary |
 Kinase Fusion gene summary Kinase Fusion gene summary |
| Kinase Fusion partner gene information | Kinase Fusion gene name: ANP32B_PRKG1 | KinaseFusionDB ID: KFG370 | FusionGDB2.0 ID: KFG370 | Hgene | Tgene | Gene symbol | ANP32B | PRKG1 | Gene ID | 10541 | 5592 | |
| Gene name | acidic nuclear phosphoprotein 32 family member B | protein kinase cGMP-dependent 1 | ||||||||||
| Synonyms | APRIL|PHAPI2|SSP29 | AAT8|PKG|PKG1|PRKG1B|PRKGR1B|cGK|cGK 1|cGK1|cGKI|cGKI-BETA|cGKI-alpha | ||||||||||
| Cytomap | 9q22.33 | 10q11.23-q21.1 | ||||||||||
| Type of gene | protein-coding | protein-coding | ||||||||||
| Description | acidic leucine-rich nuclear phosphoprotein 32 family member Bacidic (leucine-rich) nuclear phosphoprotein 32 family, member Bacidic protein rich in leucinesputative HLA-DR-associated protein I-2silver-stainable protein SSP29 | cGMP-dependent protein kinase 1protein kinase, cGMP-dependent, regulatory, type I, betaprotein kinase, cGMP-dependent, type I | ||||||||||
| Modification date | 20240310 | 20240411 | ||||||||||
| UniProtAcc | Q92688 | Q13976 | ||||||||||
| Ensembl transtripts involved in fusion gene | ENST ids | ENST00000339399, ENST00000473205, | ENST00000373975, ENST00000373980, ENST00000373985, ENST00000401604, | |||||||||
| Context (manual curation of fusion genes in KinaseFusionDB) | PubMed: ANP32B [Title/Abstract] AND PRKG1 [Title/Abstract] AND fusion [Title/Abstract] | |||||||||||
| Most frequent breakpoint (based on all fusion genes of FusionGDB 2.0) | ANP32B(100745891)-PRKG1(53814244), # samples:1 | |||||||||||
 Gene ontology of each fusion partner gene with evidence of Inferred from Direct Assay (IDA) from Entrez Gene ontology of each fusion partner gene with evidence of Inferred from Direct Assay (IDA) from Entrez |
| Partner | Gene | GO ID | GO term | PubMed ID |
| Hgene | ANP32B | GO:0006334 | nucleosome assembly | 20538007 |
| Hgene | ANP32B | GO:0045596 | negative regulation of cell differentiation | 22705300 |
| Tgene | PRKG1 | GO:0006468 | protein phosphorylation | 15905169 |
| Tgene | PRKG1 | GO:1904706 | negative regulation of vascular associated smooth muscle cell proliferation | 25447536 |
| Tgene | PRKG1 | GO:1904753 | negative regulation of vascular associated smooth muscle cell migration | 25447536 |
 Kinase Fusion gene breakpoints across ANP32B (5'-gene) Kinase Fusion gene breakpoints across ANP32B (5'-gene)* Click on the image to open the UCSC genome browser with custom track showing this image in a new window. |
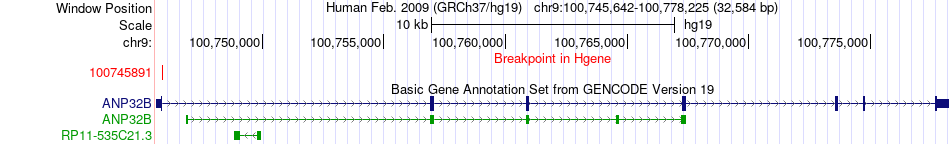 |
 Kinase Fusion gene breakpoints across PRKG1 (3'-gene) Kinase Fusion gene breakpoints across PRKG1 (3'-gene)* Click on the image to open the UCSC genome browser with custom track showing this image in a new window. |
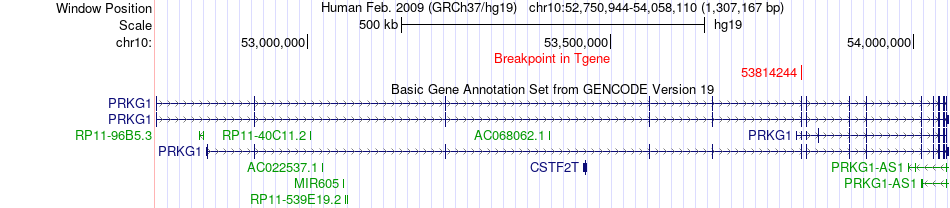 |
Top |
Kinase Fusion Gene Sample Information |
 Kinase Fusion gene information. Kinase Fusion gene information. |
 Kinase Fusion gene information from four resources (ChiTars 5.0, ChimerDB 4.0, COSMIC, and CCLE) Kinase Fusion gene information from four resources (ChiTars 5.0, ChimerDB 4.0, COSMIC, and CCLE)* All genome coordinats were lifted-over on hg19. * Click on the break point to see the gene structure around the break point region using the UCSC Genome Browser. |
| Source | Sample | Hgene | Hchr | Hbp | Tgene | Tchr | Tbp |
| ChimerDB4 | TCGA-13-0720-01A | ANP32B | chr9 | 100745891 | PRKG1 | chr10 | 53814244 |
Top |
Kinase Fusion ORF Analysis |
 Kinase Fusion information from ORFfinder translation from full-length transcript sequence from KinaseFusionDB. Kinase Fusion information from ORFfinder translation from full-length transcript sequence from KinaseFusionDB. |
| Henst | Tenst | Hgene | Hchr | Hbp | Tgene | Tchr | Tbp | Seq length (transcript) | Seq length (amino acids) |
| ENST00000339399 | ENST00000401604 | ANP32B | chr9 | 100745891 | PRKG1 | chr10 | 53814244 | 3162 | 501 |
Top |
Kinase Fusion Amino Acid Sequences |
 For individual full-length fusion transcript sequence from KinaseFusionDB, we ran ORFfinder and chose the longest ORF among the all predicted ones. For individual full-length fusion transcript sequence from KinaseFusionDB, we ran ORFfinder and chose the longest ORF among the all predicted ones. |
| >Henst_Tenst_Hgene_Hchr_Hbp_Tgene_Tchr_Tbp_length(fusion AA)_AAseq >ENST00000339399_ENST00000401604_ANP32B_chr9_100745891_PRKG1_chr10_53814244_length(amino acids)=501 MPARRPPPRTAPPASAASKPFRRPSLRKPGRLSPLRPRRGKLSLKRGEEGNMDMKRRIHLELRNRTPAATHYENGEYIIRQGARGDTFFI ISKGTVNVTREDSPSEDPVFLRTLGKGDWFGEKALQGEDVRTANVIAAEAVTCLVIDRDSFKHLIGGLDDVSNKAYEDAEAKAKYEAEAA FFANLKLSDFNIIDTLGVGGFGRVELVQLKSEESKTFAMKILKKRHIVDTRQQEHIRSEKQIMQGAHSDFIVRLYRTFKDSKYLYMLMEA CLGGELWTILRDRGSFEDSTTRFYTACVVEAFAYLHSKGIIYRDLKPENLILDHRGYAKLVDFGFAKKIGFGKKTWTFCGTPEYVAPEII LNKGHDISADYWSLGILMYELLTGSPPFSGPDPMKTYNIILRGIDMIEFPKKIAKNAANLIKKLCRDNPSERLGNLKNGVKDIQKHKWFE -------------------------------------------------------------- |
Multiple Sequence Alignment of All Fusion Protein Isoforms |
Top |
Kinase Fusion Protein Functional Features |
 Four levels of functional features of fusion genes Four levels of functional features of fusion genesGo to FGviewer search page for the most frequent breakpoint (https://ccsmweb.uth.edu/FGviewer/chr9:100745891/chr10:53814244) - FGviewer provides the online visualization of the retention search of the protein functional features across DNA, RNA, protein, and pathological levels. - How to search 1. Put your fusion gene symbol. 2. Press the tab key until there will be shown the breakpoint information filled. 4. Go down and press 'Search' tab twice. 4. Go down to have the hyperlink of the search result. 5. Click the hyperlink. 6. See the FGviewer result for your fusion gene. |
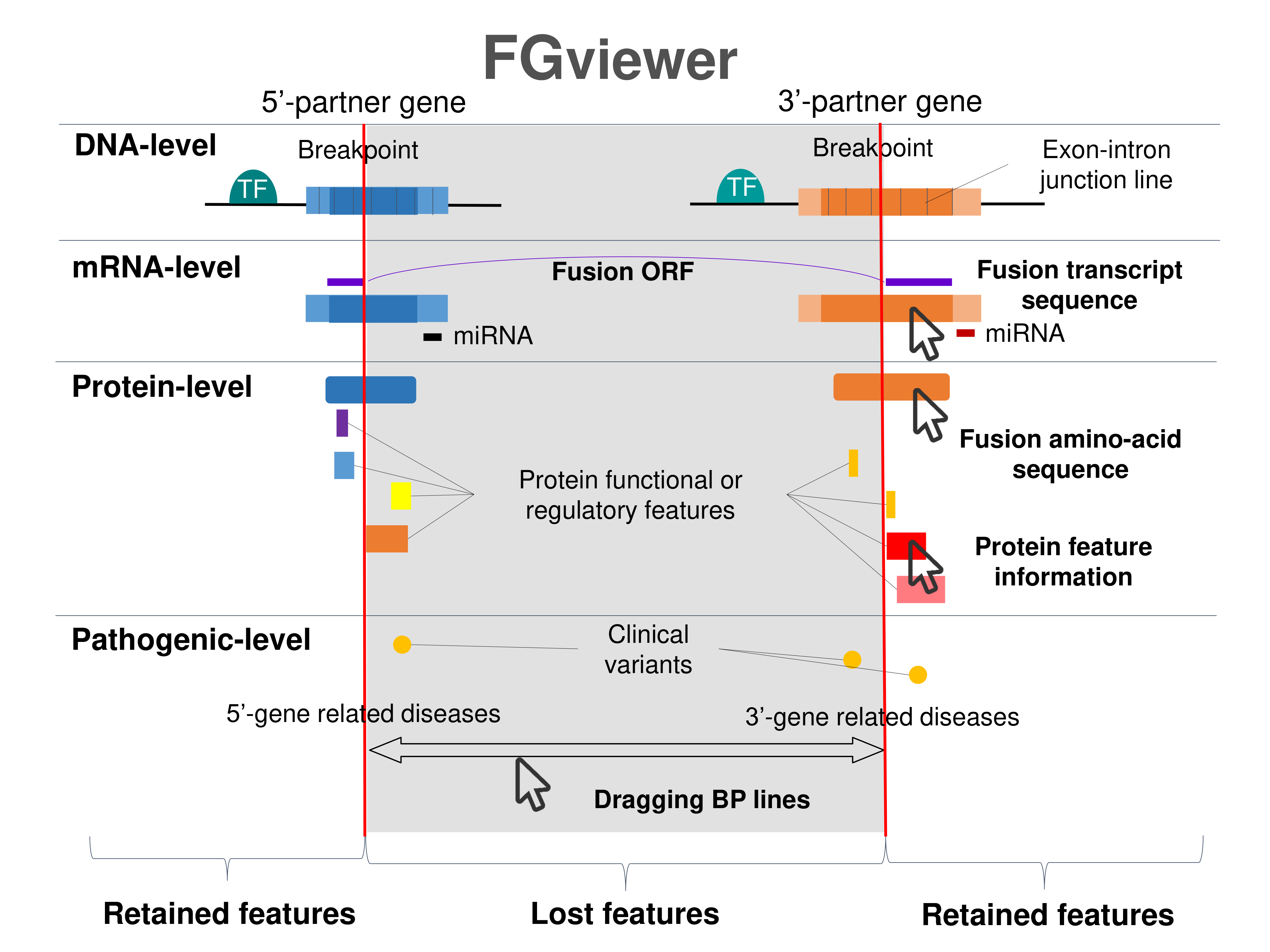 |
 Main function of each fusion partner protein. (from UniProt) Main function of each fusion partner protein. (from UniProt) |
| Hgene | Tgene |
| ANP32B | PRKG1 |
| FUNCTION: Multifunctional protein that is involved in the regulation of many processes including cell proliferation, apoptosis, cell cycle progression or transcription (PubMed:20015864, PubMed:18039846). Regulates the proliferation of neuronal stem cells, differentiation of leukemic cells and progression from G1 to S phase of the cell cycle. As negative regulator of caspase-3-dependent apoptosis, may act as an antagonist of ANP32A in regulating tissue homeostasis (PubMed:20015864). Exhibits histone chaperone properties, able to recruit histones to certain promoters, thus regulating the transcription of specific genes (PubMed:20538007, PubMed:18039846). Also plays an essential role in the nucleocytoplasmic transport of specific mRNAs via the uncommon nuclear mRNA export receptor XPO1/CRM1 (PubMed:17178712). Participates in the regulation of adequate adaptive immune responses by acting on mRNA expression and cell proliferation (By similarity). {ECO:0000250|UniProtKB:Q9EST5, ECO:0000269|PubMed:17178712, ECO:0000269|PubMed:18039846, ECO:0000269|PubMed:20015864, ECO:0000269|PubMed:20538007}.; FUNCTION: (Microbial infection) Plays an essential role in influenza A and B viral genome replication (PubMed:33045004, PubMed:31217244). Also plays a role in foamy virus mRNA export from the nucleus to the cytoplasm (PubMed:21159877). {ECO:0000269|PubMed:21159877, ECO:0000269|PubMed:31217244, ECO:0000269|PubMed:33045004}. | FUNCTION: Serine/threonine protein kinase that acts as a key mediator of the nitric oxide (NO)/cGMP signaling pathway. GMP binding activates PRKG1, which phosphorylates serines and threonines on many cellular proteins. Numerous protein targets for PRKG1 phosphorylation are implicated in modulating cellular calcium, but the contribution of each of these targets may vary substantially among cell types. Proteins that are phosphorylated by PRKG1 regulate platelet activation and adhesion, smooth muscle contraction, cardiac function, gene expression, feedback of the NO-signaling pathway, and other processes involved in several aspects of the CNS like axon guidance, hippocampal and cerebellar learning, circadian rhythm and nociception. Smooth muscle relaxation is mediated through lowering of intracellular free calcium, by desensitization of contractile proteins to calcium, and by decrease in the contractile state of smooth muscle or in platelet activation. Regulates intracellular calcium levels via several pathways: phosphorylates IRAG1 and inhibits IP3-induced Ca(2+) release from intracellular stores, phosphorylation of KCNMA1 (BKCa) channels decreases intracellular Ca(2+) levels, which leads to increased opening of this channel. PRKG1 phosphorylates the canonical transient receptor potential channel (TRPC) family which inactivates the associated inward calcium current. Another mode of action of NO/cGMP/PKGI signaling involves PKGI-mediated inactivation of the Ras homolog gene family member A (RhoA). Phosphorylation of RHOA by PRKG1 blocks the action of this protein in myriad processes: regulation of RHOA translocation; decreasing contraction; controlling vesicle trafficking, reduction of myosin light chain phosphorylation resulting in vasorelaxation. Activation of PRKG1 by NO signaling alters also gene expression in a number of tissues. In smooth muscle cells, increased cGMP and PRKG1 activity influence expression of smooth muscle-specific contractile proteins, levels of proteins in the NO/cGMP signaling pathway, down-regulation of the matrix proteins osteopontin and thrombospondin-1 to limit smooth muscle cell migration and phenotype. Regulates vasodilator-stimulated phosphoprotein (VASP) functions in platelets and smooth muscle. {ECO:0000269|PubMed:10567269, ECO:0000269|PubMed:11162591, ECO:0000269|PubMed:11723116, ECO:0000269|PubMed:12082086, ECO:0000269|PubMed:14608379, ECO:0000269|PubMed:15194681, ECO:0000269|PubMed:16990611, ECO:0000269|PubMed:8182057}. |
 Retention analysis result of each fusion partner protein across 39 protein features of UniProt such as six molecule processing features, 13 region features, four site features, six amino acid modification features, two natural variation features, five experimental info features, and 3 secondary structure features. Here, because of limited space for viewing, we only show the protein feature retention information belong to the 13 regional features. All retention annotation result can be downloaded at * Minus value of BPloci means that the break pointn is located before the CDS. Retention analysis result of each fusion partner protein across 39 protein features of UniProt such as six molecule processing features, 13 region features, four site features, six amino acid modification features, two natural variation features, five experimental info features, and 3 secondary structure features. Here, because of limited space for viewing, we only show the protein feature retention information belong to the 13 regional features. All retention annotation result can be downloaded at * Minus value of BPloci means that the break pointn is located before the CDS. |
 - Retained domain in the 5'-partner of fusion protein (protein functional feature from UniProt). - Retained domain in the 5'-partner of fusion protein (protein functional feature from UniProt). |
| Partner | Hgeneene | Hbp | Tgeneene | Tbp | ENST | BPexon | TotalExon | Protein feature loci | BPloci | TotalLen | Feature | Note |
 - Retained domain in the 3'-partner of fusion protein (protein functional feature from UniProt). - Retained domain in the 3'-partner of fusion protein (protein functional feature from UniProt). |
| Partner | Hgeneene | Hbp | Tgeneene | Tbp | ENST | BPexon | TotalExon | Protein feature loci | BPloci | TotalLen | Feature | Note |
| Tgene | ANP32B | 100745891 | PRKG1 | 53814244 | ENST00000339399 | 4 | 18 | 620_671 | 239 | 672 | Domain | Note=AGC-kinase C-terminal;Ontology_term=ECO:0000255;evidence=ECO:0000255|PROSITE-ProRule:PRU00618 |
| Tgene | ANP32B | 100745891 | PRKG1 | 53814244 | ENST00000339399 | 4 | 18 | 620_671 | 254 | 687 | Domain | Note=AGC-kinase C-terminal;Ontology_term=ECO:0000255;evidence=ECO:0000255|PROSITE-ProRule:PRU00618 |
| Tgene | ANP32B | 100745891 | PRKG1 | 53814244 | ENST00000339399 | 4 | 18 | 360_619 | 239 | 672 | Domain | Note=Protein kinase;Ontology_term=ECO:0000255;evidence=ECO:0000255|PROSITE-ProRule:PRU00159 |
| Tgene | ANP32B | 100745891 | PRKG1 | 53814244 | ENST00000339399 | 4 | 18 | 360_619 | 254 | 687 | Domain | Note=Protein kinase;Ontology_term=ECO:0000255;evidence=ECO:0000255|PROSITE-ProRule:PRU00159 |
Top |
Kinase Fusion Protein Structures |
 CIF files of the predicted kinase fusion proteins CIF files of the predicted kinase fusion proteins * Here we show the 3D structure of the fusion proteins using Mol*. AlphaFold produces a per-residue confidence score (pLDDT) between 0 and 100. Model confidence is shown from the pLDDT values per residue. pLDDT corresponds to the model’s prediction of its score on the local Distance Difference Test. It is a measure of local accuracy (from AlphfaFold website). To color code individual residues, we transformed individual PDB files into CIF format. |
| Kinase Fusion protein CIF link (fusion AA seq ID in KinaseFusionDB) | Henst | Tenst | Hgene | Hchr | Hbp | Tgene | Tchr | Tbp | AA seq | Len(AA seq) |
| PDB file >>>25_ANP32B_PRKG1 | ENST00000339399 | ENST00000401604 | ANP32B | chr9 | 100745891 | PRKG1 | chr10 | 53814244 | MPARRPPPRTAPPASAASKPFRRPSLRKPGRLSPLRPRRGKLSLKRGEEGNMDMKRRIHLELRNRTPAATHYENGEYIIRQGARGDTFFI ISKGTVNVTREDSPSEDPVFLRTLGKGDWFGEKALQGEDVRTANVIAAEAVTCLVIDRDSFKHLIGGLDDVSNKAYEDAEAKAKYEAEAA FFANLKLSDFNIIDTLGVGGFGRVELVQLKSEESKTFAMKILKKRHIVDTRQQEHIRSEKQIMQGAHSDFIVRLYRTFKDSKYLYMLMEA CLGGELWTILRDRGSFEDSTTRFYTACVVEAFAYLHSKGIIYRDLKPENLILDHRGYAKLVDFGFAKKIGFGKKTWTFCGTPEYVAPEII LNKGHDISADYWSLGILMYELLTGSPPFSGPDPMKTYNIILRGIDMIEFPKKIAKNAANLIKKLCRDNPSERLGNLKNGVKDIQKHKWFE | 501 |
| 3D view using mol* of 25_ANP32B_PRKG1 | ||||||||||
| PDB file >>>TKFP_40_ANP32B_PRKG1 | ENST00000339399 | ENST00000401604 | ANP32B | chr9 | 100745891 | PRKG1 | chr10 | 53814244 | MPARRPPPRTAPPASAASKPFRRPSLRKPGRLSPLRPRRGKLSLKRGEEGNMDMKRRIHLELRNRTPAATHYENGEYIIRQGARGDTFFI ISKGTVNVTREDSPSEDPVFLRTLGKGDWFGEKALQGEDVRTANVIAAEAVTCLVIDRDSFKHLIGGLDDVSNKAYEDAEAKAKYEAEAA FFANLKLSDFNIIDTLGVGGFGRVELVQLKSEESKTFAMKILKKRHIVDTRQQEHIRSEKQIMQGAHSDFIVRLYRTFKDSKYLYMLMEA CLGGELWTILRDRGSFEDSTTRFYTACVVEAFAYLHSKGIIYRDLKPENLILDHRGYAKLVDFGFAKKIGFGKKTWTFCGTPEYVAPEII LNKGHDISADYWSLGILMYELLTGSPPFSGPDPMKTYNIILRGIDMIEFPKKIAKNAANLIKKLCRDNPSERLGNLKNGVKDIQKHKWFE | 501_ANP32B_PRKG1 |
Top |
Comparison of Fusion Protein Isoforms |
 Superimpose the 3D Structures Among All Fusion Protein Isoforms Superimpose the 3D Structures Among All Fusion Protein Isoforms * Download the pdb file and open it from the molstar online viewer. |
 Comparison of the Secondary Structures of Fusion Protein Isoforms Comparison of the Secondary Structures of Fusion Protein Isoforms |
Top |
Comparison of Fusion Protein Sequences/Structures with Known Sequences/Structures from PDB |
Top |
pLDDT score distribution |
 pLDDT score distribution of the predicted fusion protein structures from AlphaFold2 pLDDT score distribution of the predicted fusion protein structures from AlphaFold2* AlphaFold produces a per-residue confidence score (pLDDT) between 0 and 100. * The blue color at the bottom marks the best active site residues. |
| 25_ANP32B_PRKG1.png |
 |
| 25_ANP32B_PRKG1.png |
 |
Top |
Potential Active Site Information |
 The potential binding sites of these fusion proteins were identified using SiteMap, a module of the Schrodinger suite. The potential binding sites of these fusion proteins were identified using SiteMap, a module of the Schrodinger suite. |
| Kinase Fusion AA seq ID in KinaseFusionDB | Site score | Size | Dscore | Volume | Exposure | Enclosure | Contact | Phobic | Philic | Balance | Don/Acc | Residues |
Top |
Ramachandran Plot of Kinase Fusion Protein Structure |
 Ramachandran plot of the torsional angles - phi (φ)and psi (ψ) - of the residues (amino acids) contained in this fusion protein peptide. Ramachandran plot of the torsional angles - phi (φ)and psi (ψ) - of the residues (amino acids) contained in this fusion protein peptide. |
| 25_ANP32B_PRKG1_ramachandran.png |
 |
Top |
Virtual Screening Results |
 Distribution of the average docking score across all approved kinase inhibitors. Distribution of the average docking score across all approved kinase inhibitors.Distribution of the number of occurrence across all approved kinase inhibitors. |
| 5'-kinase fusion protein case |
| 3'-kinase fusion protein case |
 |
Top |
 Drug information from DrugBank of the top 20 interacting small molecules. Drug information from DrugBank of the top 20 interacting small molecules.* The detailed information of individual kinase inhibitors are available in the download page. |
| Fusion gene name info | Drug | Docking score | Glide g score | Glide energy |
| 25_ANP32B_PRKG1-DOCK_HTVS_1-001 | Sunitinib | -7.29282 | -7.29702 | -43.6323 |
| 25_ANP32B_PRKG1-DOCK_HTVS_1-001 | Abrocitinib | -6.33824 | -6.34934 | -37.5673 |
| 25_ANP32B_PRKG1-DOCK_HTVS_1-001 | Abrocitinib | -6.33824 | -6.34934 | -37.5673 |
| 25_ANP32B_PRKG1-DOCK_HTVS_1-001 | Crizotinib | -6.3275 | -6.8234 | -46.2289 |
| 25_ANP32B_PRKG1-DOCK_HTVS_1-001 | Crizotinib | -6.3275 | -6.8234 | -46.2289 |
| 25_ANP32B_PRKG1-DOCK_HTVS_1-001 | Netarsudil | -6.0894 | -6.1005 | -7.830369999999999 |
| 25_ANP32B_PRKG1-DOCK_HTVS_1-001 | Netarsudil | -6.0894 | -6.1005 | -7.830369999999999 |
| 25_ANP32B_PRKG1-DOCK_HTVS_1-001 | Netarsudil | -5.86908 | -5.88018 | -50.6219 |
| 25_ANP32B_PRKG1-DOCK_HTVS_1-001 | Netarsudil | -5.86908 | -5.88018 | -50.6219 |
| 25_ANP32B_PRKG1-DOCK_HTVS_1-001 | Sunitinib | -5.827459999999999 | -5.83166 | -41.928999999999995 |
| 25_ANP32B_PRKG1-DOCK_HTVS_1-001 | Asciminib | -5.76805 | -6.13115 | -25.0666 |
| 25_ANP32B_PRKG1-DOCK_HTVS_1-001 | Afatinib | -5.6507 | -5.832999999999999 | -40.7201 |
| 25_ANP32B_PRKG1-DOCK_HTVS_1-001 | Afatinib | -5.6507 | -5.832999999999999 | -40.7201 |
| 25_ANP32B_PRKG1-DOCK_HTVS_1-001 | Afatinib | -5.6493 | -5.832999999999999 | -40.7201 |
| 25_ANP32B_PRKG1-DOCK_HTVS_1-001 | Crizotinib | -5.55661 | -5.89281 | -46.2839 |
| 25_ANP32B_PRKG1-DOCK_HTVS_1-001 | Crizotinib | -5.55661 | -5.89281 | -46.2839 |
| 25_ANP32B_PRKG1-DOCK_HTVS_1-001 | Tofacitinib | -5.4407 | -5.4522 | -17.2654 |
| 25_ANP32B_PRKG1-DOCK_HTVS_1-001 | Tofacitinib | -5.4407 | -5.4522 | -17.2654 |
| 25_ANP32B_PRKG1-DOCK_HTVS_1-001 | Upadacitinib | -5.3943 | -5.3953 | -28.074 |
| 25_ANP32B_PRKG1-DOCK_HTVS_1-001 | Crizotinib | -5.32501 | -5.66121 | -23.5325 |
Top |
Kinase-Substrate Information of ANP32B_PRKG1 |
 Phosphorylation target of the kinase Phosphorylation target of the kinase(phosphosite, 03-17-2024) |
| Kinase | Kinase UniProt Acc | Kinase species | Substrate | Substrate UniProt Acc | Substrate phosphorylated residues | Substrate phosphorylated sites (+/-7AA) | Domain |
| PRKG1 | Q13976-2 | human | HSPB1 | P04792 | T143 | RCFtRkytLPPGVDP | HSP20 |
| PRKG1 | Q13976-2 | human | HSPB1 | P04792 | S15 | FsLLrGPsWDPFrDW | |
| PRKG1 | Q13976-2 | human | PRKG1 | Q13976-2 | S65 | TQQAQKQsASTLQGE | |
| PRKG1 | Q13976-2 | human | HSPB1 | P04792 | S82 | RALsRQLssGVsEIr | |
| PRKG1 | Q13976-2 | human | HSPB1 | P04792 | S78 | PAysRALsRQLssGV | |
| PRKG1 | Q13976-2 | human | PRKG1 | Q13976-2 | S81 | RtKRQAIsAEPTAFD | |
| PRKG1 | Q13976-2 | human | PAK1 | Q13153 | S21 | APPMRNtsTMIGAGs | |
| PRKG1 | Q13976-2 | human | LASP1 | Q14847 | S146 | MEPERRDsQDGssyR | |
| PRKG1 | Q13976 | human | GATA4 | P43694 | S262 | IkPQRRLsAsRRVGL | |
| PRKG1 | Q13976 | human | PRKAR1A | P10644 | S101 | RRRRGAIsAEVYTEE | |
| PRKG1 | Q13976 | human | SRF | P11831 | T159 | DNkLRRYtTFsKRkT | SRF-TF |
| PRKG1 | Q13976 | human | VASP | P50552 | T278 | LARRRKAtQVGEktP | |
| PRKG1 | Q13976 | human | TBXA2R | P21731-2 | T332 | TRPRRsLtLWPSLEY | |
| PRKG1 | Q13976 | human | CBS | P35520 | S227 | LDQYRNAsNPLAHYD | PALP |
| PRKG1 | Q13976 | human | TRPC6 | Q9Y210 | T70 | RLAHRRQtVLREKGR | |
| PRKG1 | Q13976 | human | HRH1 | P35367 | S398 | WKRLRsHsRQyVSGL | 7tm_1 |
| PRKG1 | Q13976 | human | RGS18 | Q9NS28 | S216 | PTNLRRRsRsFTCNE | |
| PRKG1 | Q13976 | human | TBXA2R | P21731-2 | S330 | LSTRPRRsLtLWPSL | |
| PRKG1 | Q13976 | human | ENSA | O43768 | S109 | DLPQRkssLVtskLA | Endosulfine |
| PRKG1 | Q13976 | human | FHOD1 | Q9Y613 | S1131 | AARERKRsRGNRKsL | |
| PRKG1 | Q13976 | human | GTF2I | P78347 | S784 | GVPFRRPstFGIPRL | GTF2I |
| PRKG1 | Q13976 | human | ORAI1 | Q96D31 | S34 | sRRsRRRsGDGEPPG | |
| PRKG1 | Q13976 | human | PPP1R14A | Q96A00 | T38 | QkRHARVtVkYDRRE | PP1_inhibitor |
| PRKG1 | Q13976 | human | TRPC3 | Q13507-3 | T11 | SPSLRRMtVMREKGR | |
| PRKG1 | Q13976 | human | RAP1GAP2 | Q684P5 | S7 | _MFGRKRsVsFGGFG | |
| PRKG1 | Q13976 | human | STMN1 | P16949 | S63 | AAEERRksHEAEVLk | Stathmin |
| PRKG1 | Q13976 | human | RGS2 | P41220 | S46 | kDWKTRLsYFLQNSS | |
| PRKG1 | Q13976 | human | TTN | Q8WZ42 | S4099 | sEQPGLFsEWLRNIE | |
| PRKG1 | Q13976 | human | TBXA2R | P21731 | S331 | sTRPRsLsLQPQLtQ | |
| PRKG1 | Q13976 | human | PRKG1 | Q13976 | S65 | TtRAQGIsAEPQTYR | |
| PRKG1 | Q13976 | human | GTF2I | P78347 | S412 | GIPFRRPstYGIPRL | GTF2I |
| PRKG1 | Q13976 | human | RGS2 | P41220 | S64 | kPKTGKKsKQQAFIk | |
| PRKG1 | Q13976 | human | TRPC1 | P48995 | S206 | SAKNKKDsLRHSRFR | TRP_2 |
| PRKG1 | Q13976 | human | CCDC80 | Q76M96 | S434 | PQTTRRPsKATSLES | |
| PRKG1 | Q13976 | human | HRH1 | P35367 | S396 | FTWKRLRsHsRQyVS | 7tm_1 |
| PRKG1 | Q13976 | human | ARPP19 | P56211 | S104 | DLPQRkPsLVAskLA | Endosulfine |
| PRKG1 | Q13976 | human | CCDC80 | Q76M96 | S409 | VITARRPsVsENLYP | |
| PRKG1 | Q13976 | human | PDE5A | O76074 | S102 | GTPTRKIsAsEFDRP | |
| PRKG1 | Q13976 | human | VASP | P50552 | S239 | GAKLRKVsKQEEASG | |
| PRKG1 | Q13976 | human | RAP1B | P61224 | S179 | PGkARKKsscQLL__ | |
| PRKG1 | Q13976 | human | RHOA | P61586 | S188 | ARRGKKKsGCLVL__ | |
| PRKG1 | Q13976 | human | LASP1 | Q14847 | S146 | MEPERRDsQDGssyR | |
| PRKG1 | Q13976 | human | TRPC6 | Q9Y210 | S322 | KNDYKKLsMQCKDFV | |
| PRKG1 | Q13976 | human | TTN | Q8WZ42 | S4185 | IQQGAKtsLQEEMDs | |
| PRKG1 | Q13976 | human | LIPE | Q05469 | S853 | IAEPMRRsVsEAALA | |
| PRKG1 | Q13976 | human | TSC2 | P49815 | S1364 | IPIERVVssEGGRPs | |
| PRKG1 | Q13976 | human | TRPC1 | P48995 | T347 | SGYRRKPtCKKIMTV | |
| PRKG1 | Q13976 | human | GTF2I | P78347-2 | S743 | GVPFRRPstFGIPRL | GTF2I |
| PRKG1 | Q13976 | human | SLC6A4 | P31645 | T276 | SIWKGVKtsGKVVWV | SNF |
| PRKG1 | Q13976 | human | TTN | Q8WZ42 | S4092 | ALQAAVAsEQPGLFs | |
| PRKG1 | Q13976 | human | TRPC3 | Q13507 | S336 | KNDYRKLsMQCKDFV | |
| PRKG1 | Q13976 | human | PTS | Q03393 | S19 | AQVSRRIsFSASHRL | PTPS |
| PRKG1 | Q13976 | human | CBS | P35520 | S525 | YHSTGKSsQRQMVFG | |
| PRKG1 | Q13976 | human | ARHGEF6 | Q15052 | S684 | GSSTRkDsIPQVLLP | |
| PRKG1 | Q13976 | human | TTN | Q8WZ42 | S4010 | VRIEEGKsLRFPLAL | |
| PRKG1 | Q13976 | human | VASP | P50552 | S157 | EHIERRVsNAGGPPA | |
| PRKG1 | Q13976 | human | TTN | Q8WZ42 | S11878 | KEEVVLKsVLRKRPE | |
| PRKG1 | Q13976 | human | GTF2I | P78347-2 | S371 | GIPFRRPsTYGIPRL | GTF2I |
 Biological Network Integration of This Kinase and Substrates Biological Network Integration of This Kinase and Substrates (GeneMANIA website) |
 Enriched GO biological processes of the phosphorylation target genes of the kinase Enriched GO biological processes of the phosphorylation target genes of the kinase |
| Kinase | GOID | GO term | P.adjust |
| PRKG1 | ID | Description | 0.00e+00 |
| PRKG1 | GO:0035296 | regulation of tube diameter | 3.05e-06 |
| PRKG1 | GO:0097746 | blood vessel diameter maintenance | 3.05e-06 |
| PRKG1 | GO:0035150 | regulation of tube size | 3.05e-06 |
| PRKG1 | GO:0090257 | regulation of muscle system process | 3.50e-06 |
| PRKG1 | GO:0006937 | regulation of muscle contraction | 4.78e-06 |
| PRKG1 | GO:0003300 | cardiac muscle hypertrophy | 4.78e-06 |
| PRKG1 | GO:0014897 | striated muscle hypertrophy | 4.83e-06 |
| PRKG1 | GO:0014896 | muscle hypertrophy | 4.83e-06 |
| PRKG1 | GO:0003012 | muscle system process | 9.44e-06 |
| PRKG1 | GO:0031032 | actomyosin structure organization | 9.44e-06 |
| PRKG1 | GO:0006940 | regulation of smooth muscle contraction | 9.44e-06 |
| PRKG1 | GO:0006936 | muscle contraction | 1.76e-05 |
| PRKG1 | GO:0007584 | response to nutrient | 3.10e-05 |
| PRKG1 | GO:1903522 | regulation of blood circulation | 3.10e-05 |
| PRKG1 | GO:0003018 | vascular process in circulatory system | 3.68e-05 |
| PRKG1 | GO:0055013 | cardiac muscle cell development | 3.68e-05 |
| PRKG1 | GO:0055006 | cardiac cell development | 4.85e-05 |
| PRKG1 | GO:0042060 | wound healing | 4.85e-05 |
| PRKG1 | GO:0055001 | muscle cell development | 8.42e-05 |
| PRKG1 | GO:0006939 | smooth muscle contraction | 8.72e-05 |
| PRKG1 | GO:0045933 | positive regulation of muscle contraction | 8.72e-05 |
| PRKG1 | GO:0030038 | contractile actin filament bundle assembly | 8.72e-05 |
| PRKG1 | GO:0043149 | stress fiber assembly | 8.72e-05 |
| PRKG1 | GO:0055007 | cardiac muscle cell differentiation | 1.98e-04 |
| PRKG1 | GO:0045936 | negative regulation of phosphate metabolic process | 2.10e-04 |
| PRKG1 | GO:0010563 | negative regulation of phosphorus metabolic process | 2.10e-04 |
| PRKG1 | GO:0048738 | cardiac muscle tissue development | 2.33e-04 |
| PRKG1 | GO:0014706 | striated muscle tissue development | 3.29e-04 |
| PRKG1 | GO:0045932 | negative regulation of muscle contraction | 4.28e-04 |
| PRKG1 | GO:0051017 | actin filament bundle assembly | 4.33e-04 |
| PRKG1 | GO:0060537 | muscle tissue development | 4.35e-04 |
| PRKG1 | GO:0035051 | cardiocyte differentiation | 4.44e-04 |
| PRKG1 | GO:0061572 | actin filament bundle organization | 4.44e-04 |
| PRKG1 | GO:0042310 | vasoconstriction | 5.71e-04 |
| PRKG1 | GO:0035265 | organ growth | 5.71e-04 |
| PRKG1 | GO:0051146 | striated muscle cell differentiation | 5.71e-04 |
| PRKG1 | GO:0007015 | actin filament organization | 5.71e-04 |
| PRKG1 | GO:0055017 | cardiac muscle tissue growth | 6.71e-04 |
| PRKG1 | GO:0003298 | physiological muscle hypertrophy | 6.71e-04 |
| PRKG1 | GO:0003301 | physiological cardiac muscle hypertrophy | 6.71e-04 |
| PRKG1 | GO:0045987 | positive regulation of smooth muscle contraction | 6.71e-04 |
| PRKG1 | GO:0061049 | cell growth involved in cardiac muscle cell development | 6.71e-04 |
| PRKG1 | GO:0031667 | response to nutrient levels | 7.29e-04 |
| PRKG1 | GO:0051492 | regulation of stress fiber assembly | 7.29e-04 |
| PRKG1 | GO:0045907 | positive regulation of vasoconstriction | 7.60e-04 |
| PRKG1 | GO:0060562 | epithelial tube morphogenesis | 7.60e-04 |
| PRKG1 | GO:0060419 | heart growth | 7.72e-04 |
| PRKG1 | GO:0090075 | relaxation of muscle | 8.61e-04 |
| PRKG1 | GO:0110020 | regulation of actomyosin structure organization | 1.01e-03 |
Top |
Related Drugs to ANP32B_PRKG1 |
 Drugs used for this fusion-positive patient. Drugs used for this fusion-positive patient. (Manual curation of PubMed, 04-30-2022 + MyCancerGenome) |
| Hgene | Tgene | Drug | Source | PMID |
 Distribution of the number of studies mentioning ANP32B-PRKG1 and kinase inhibitors the PubMed Abstract (04-01-2024) Distribution of the number of studies mentioning ANP32B-PRKG1 and kinase inhibitors the PubMed Abstract (04-01-2024) |
| Fusion gene - drug pair 1 | Fusion gene - drug pair 2 | PMID | Publication date | DOI | Study title |
Top |
Related Diseases to ANP32B_PRKG1 |
 Diseases that have this fusion gene. Diseases that have this fusion gene. (Manual curation of PubMed, 04-30-2022 + MyCancerGenome) |
| Hgene | Tgene | Disease | Source | PMID |
 Related diseases from the literature mentioned this fusion gene and drug. Related diseases from the literature mentioned this fusion gene and drug. (PubMed, 04-01-2024) |
| MeSH ID | MeSH term |
 Diseases associated with fusion partners. Diseases associated with fusion partners. (DisGeNet 4.0) |
| Partner | Gene | Disease ID | Disease name | # pubmeds | Source |
Top |
Clinical Trials of the Found Drugs/Small Molecules |
 Statistics of the Clinical Trials of the Found Kinase Inibitors from clinicaltrials.gov (06-17-2024) Statistics of the Clinical Trials of the Found Kinase Inibitors from clinicaltrials.gov (06-17-2024) |
 Clinical Trials from clinicaltrials.gov (06-17-2024) Clinical Trials from clinicaltrials.gov (06-17-2024) |
| Fusion Gene | Kinase Inhibitor | NCT ID | Study Status | Phases | Disease | # Enrolment | Date |

