| UTHEALTH HOME ABOUT SBMI A-Z WEBMAIL INSIDE THE UNIVERSITY |

|
|||||||
|
Kinase Fusion Gene:ANXA2_SMG1 |
Kinase Fusion Protein Summary |
 Kinase Fusion gene summary Kinase Fusion gene summary |
| Kinase Fusion partner gene information | Kinase Fusion gene name: ANXA2_SMG1 | KinaseFusionDB ID: KFG372 | FusionGDB2.0 ID: KFG372 | Hgene | Tgene | Gene symbol | ANXA2 | SMG1 | Gene ID | 302 | 23049 | |
| Gene name | annexin A2 | SMG1 nonsense mediated mRNA decay associated PI3K related kinase | ||||||||||
| Synonyms | ANX2|ANX2L4|CAL1H|HEL-S-270|LIP2|LPC2|LPC2D|P36|PAP-IV | 61E3.4|ATX|LIP | ||||||||||
| Cytomap | 15q22.2 | 16p12.3 | ||||||||||
| Type of gene | protein-coding | protein-coding | ||||||||||
| Description | annexin A2annexin IIannexin-2calpactin I heavy chaincalpactin I heavy polypeptidecalpactin-1 heavy chainchromobindin 8epididymis secretory protein Li 270epididymis secretory sperm binding proteinlipocortin IIplacental anticoagulant protein IVpr | serine/threonine-protein kinase SMG1PI-3-kinase-related kinase SMG-1SMG1 phosphatidylinositol 3-kinase-related kinaselambda-interacting proteinlambda/iota protein kinase C-interacting proteinnonsense mediated mRNA decay-associated PI3K-related kinase | ||||||||||
| Modification date | 20240411 | 20240411 | ||||||||||
| UniProtAcc | P07355 | Q96Q15 | ||||||||||
| Ensembl transtripts involved in fusion gene | ENST ids | ENST00000332680, ENST00000396024, ENST00000421017, ENST00000451270, ENST00000557937, | ENST00000565224, ENST00000567737, ENST00000389467, ENST00000446231, | |||||||||
| Context (manual curation of fusion genes in KinaseFusionDB) | PubMed: ANXA2 [Title/Abstract] AND SMG1 [Title/Abstract] AND fusion [Title/Abstract] | |||||||||||
| Most frequent breakpoint (based on all fusion genes of FusionGDB 2.0) | ANXA2(60646353)-SMG1(18883946), # samples:1 | |||||||||||
 Gene ontology of each fusion partner gene with evidence of Inferred from Direct Assay (IDA) from Entrez Gene ontology of each fusion partner gene with evidence of Inferred from Direct Assay (IDA) from Entrez |
| Partner | Gene | GO ID | GO term | PubMed ID |
| Hgene | ANXA2 | GO:0001921 | positive regulation of receptor recycling | 22848640 |
| Hgene | ANXA2 | GO:0002091 | negative regulation of receptor internalization | 18799458 |
| Hgene | ANXA2 | GO:0010756 | positive regulation of plasminogen activation | 9836589 |
| Hgene | ANXA2 | GO:0031340 | positive regulation of vesicle fusion | 2138016 |
| Hgene | ANXA2 | GO:0032804 | negative regulation of low-density lipoprotein particle receptor catabolic process | 22848640 |
| Hgene | ANXA2 | GO:0036035 | osteoclast development | 7961821 |
| Hgene | ANXA2 | GO:1905581 | positive regulation of low-density lipoprotein particle clearance | 22848640 |
| Hgene | ANXA2 | GO:1905597 | positive regulation of low-density lipoprotein particle receptor binding | 22848640 |
| Hgene | ANXA2 | GO:1905602 | positive regulation of receptor-mediated endocytosis involved in cholesterol transport | 22848640 |
| Tgene | SMG1 | GO:0000184 | nuclear-transcribed mRNA catabolic process, nonsense-mediated decay | 11544179 |
| Tgene | SMG1 | GO:0006974 | DNA damage response | 15175154 |
| Tgene | SMG1 | GO:0018105 | peptidyl-serine phosphorylation | 11544179|15175154 |
| Tgene | SMG1 | GO:0046777 | protein autophosphorylation | 11331269|11544179 |
| Tgene | SMG1 | GO:0046854 | phosphatidylinositol phosphate biosynthetic process | 11331269 |
 Kinase Fusion gene breakpoints across ANXA2 (5'-gene) Kinase Fusion gene breakpoints across ANXA2 (5'-gene)* Click on the image to open the UCSC genome browser with custom track showing this image in a new window. |
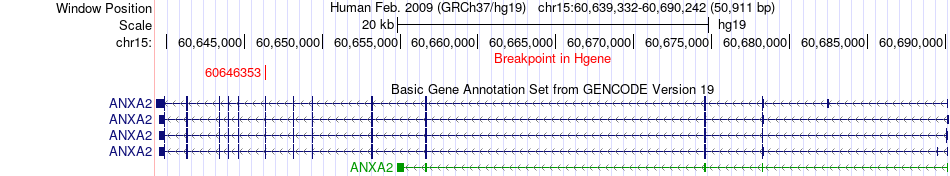 |
 Kinase Fusion gene breakpoints across SMG1 (3'-gene) Kinase Fusion gene breakpoints across SMG1 (3'-gene)* Click on the image to open the UCSC genome browser with custom track showing this image in a new window. |
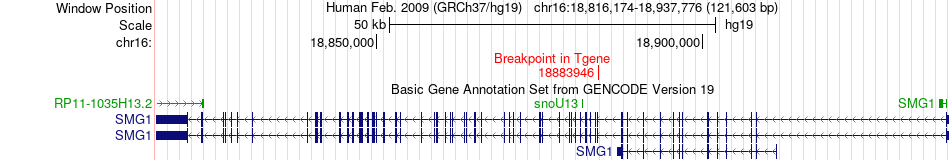 |
Top |
Kinase Fusion Gene Sample Information |
 Kinase Fusion gene information. Kinase Fusion gene information. |
 Kinase Fusion gene information from four resources (ChiTars 5.0, ChimerDB 4.0, COSMIC, and CCLE) Kinase Fusion gene information from four resources (ChiTars 5.0, ChimerDB 4.0, COSMIC, and CCLE)* All genome coordinats were lifted-over on hg19. * Click on the break point to see the gene structure around the break point region using the UCSC Genome Browser. |
| Source | Sample | Hgene | Hchr | Hbp | Tgene | Tchr | Tbp |
| ChimerDB4 | TCGA-B6-A0IQ-01A | ANXA2 | chr15 | 60646353 | SMG1 | chr16 | 18883946 |
Top |
Kinase Fusion ORF Analysis |
 Kinase Fusion information from ORFfinder translation from full-length transcript sequence from KinaseFusionDB. Kinase Fusion information from ORFfinder translation from full-length transcript sequence from KinaseFusionDB. |
| Henst | Tenst | Hgene | Hchr | Hbp | Tgene | Tchr | Tbp | Seq length (transcript) | Seq length (amino acids) |
| ENST00000396024 | ENST00000389467 | ANXA2 | chr15 | 60646353 | SMG1 | chr16 | 18883946 | 14563 | 3235 |
Top |
Kinase Fusion Amino Acid Sequences |
 For individual full-length fusion transcript sequence from KinaseFusionDB, we ran ORFfinder and chose the longest ORF among the all predicted ones. For individual full-length fusion transcript sequence from KinaseFusionDB, we ran ORFfinder and chose the longest ORF among the all predicted ones. |
| >Henst_Tenst_Hgene_Hchr_Hbp_Tgene_Tchr_Tbp_length(fusion AA)_AAseq >ENST00000396024_ENST00000389467_ANXA2_chr15_60646353_SMG1_chr16_18883946_length(amino acids)=3235 MLCASFKMSTVHEILCKLSLEGDHSTPPSAYGSVKAYTNFDAERDALNIETAIKTKGVDEVTIVNILTNRSNAQRQDIAFAYQRRTKKEL ASALKSALSGHLETVILGLLKTPAQYDASELKASMKGLGTDEDSLIEIICSRTNQELQEINRVYKEMYKTDLEKDIISDTSGDFRKLMVA LAKGRRAEDGSVIDYELIDQDARMWALSPTVFALLSKNLMIVHSDLAVHFPAIQYAVLYTLYSHCTRHDHFISSSLSSSSPSLFDGAVIS TVTTATKKHFSIILNLLGILLKKDNLNQDTRKLLMTWALEAAVLMKKSETYAPLFSLPSFHKFCKGLLANTLVEDVNICLQACSSLHALS SSLPDDLLQRCVDVCRVQLVHSGTRIRQAFGKLLKSIPLDVVLSNNNHTEIQEISLALRSHMSKAPSNTFHPQDFSDVISFILYGNSHRT GKDNWLERLFYSCQRLDKRDQSTIPRNLLKTDAVLWQWAIWEAAQFTVLSKLRTPLGRAQDTFQTIEGIIRSLAAHTLNPDQDVSQWTTA DNDEGHGNNQLRLVLLLQYLENLEKLMYNAYEGCANALTSPPKVIRTFFYTNRQTCQDWLTRIRLSIMRVGLLAGQPAVTVRHGFDLLTE MKTTSLSQGNELEVTIMMVVEALCELHCPEAIQGIAVWSSSIVGKNLLWINSVAQQAEGRFEKASVEYQEHLCAMTGVDCCISSFDKSVL TLANAGRNSASPKHSLNGESRKTVLSKPTDSSPEVINYLGNKACECYISIADWAAVQEWQNAIHDLKKSTSSTSLNLKADFNYIKSLSSF ESGKFVECTEQLELLPGENINLLAGGSKEKIDMKKLLPNMLSPDPRELQKSIEVQLLRSSVCLATALNPIEQDQKWQSITENVVKYLKQT SRIAIGPLRLSTLTVSQSLPVLSTLQLYCSSALENTVSNRLSTEDCLIPLFSEALRSCKQHDVRPWMQALRYTMYQNQLLEKIKEQTVPI RSHLMELGLTAAKFARKRGNVSLATRLLAQCSEVQLGKTTTAQDLVQHFKKLSTQGQVDEKWGPELDIEKTKLLYTAGQSTHAMEMLSSC AISFCKSVKAEYAVAKSILTLAKWIQAEWKEISGQLKQVYRAQHQQNFTGLSTLSKNILTLIELPSVNTMEEEYPRIESESTVHIGVGEP DFILGQLYHLSSVQAPEVAKSWAALASWAYRWGRKVVDNASQGEGVRLLPREKSEVQNLLPDTITEEEKERIYGILGQAVCRPAGIQDED ITLQITESEDNEEDDMVDVIWRQLISSCPWLSELDESATEGVIKVWRKVVDRIFSLYKLSCSAYFTFLKLNAGQIPLDEDDPRLHLSHRV EQSTDDMIVMATLRLLRLLVKHAGELRQYLEHGLETTPTAPWRGIIPQLFSRLNHPEVYVRQSICNLLCRVAQDSPHLILYPAIVGTISL SSESQASGNKFSTAIPTLLGNIQGEELLVSECEGGSPPASQDSNKDEPKSGLNEDQAMMQDCYSKIVDKLSSANPTMVLQVQMLVAELRR VTVLWDELWLGVLLQQHMYVLRRIQQLEDEVKRVQNNNTLRKEEKIAIMREKHTALMKPIVFALEHVRSITAAPAETPHEKWFQDNYGDA IENALEKLKTPLNPAKPGSSWIPFKEIMLSLQQRAQKRASYILRLEEISPWLAAMTNTEIALPGEVSARDTVTIHSVGGTITILPTKTKP KKLLFLGSDGKSYPYLFKGLEDLHLDERIMQFLSIVNTMFATINRQETPRFHARHYSVTPLGTRSGLIQWVDGATPLFGLYKRWQQREAA LQAQKAQDSYQTPQNPGIVPRPSELYYSKIGPALKTVGLSLDVSRRDWPLHVMKAVLEELMEATPPNLLAKELWSSCTTPDEWWRVTQSY ARSTAVMSMVGYIIGLGDRHLDNVLIDMTTGEVVHIDYNVCFEKGKSLRVPEKVPFRMTQNIETALGVTGVEGVFRLSCEQVLHIMRRGR ETLLTLLEAFVYDPLVDWTAGGEAGFAGAVYGGGGQQAESKQSKREMEREITRSLFSSRVAEIKVNWFKNRDEMLVVLPKLDGSLDEYLS LQEQLTDVEKLQGKLLEEIEFLEGAEGVDHPSHTLQHRYSEHTQLQTQQRAVQEAIQVKLNEFEQWITHYQAAFNNLEATQLASLLQEIS TQMDLGPPSYVPATAFLQNAGQAHLISQCEQLEGEVGALLQQRRSVLRGCLEQLHHYATVALQYPKAIFQKHRIEQWKTWMEELICNTTV ERCQELYRKYEMQYAPQPPPTVCQFITATEMTLQRYAADINSRLIRQVERLKQEAVTVPVCEDQLKEIERCIKVFLHENGEEGSLSLASV IISALCTLTRRNLMMEGAASSAGEQLVDLTSRDGAWFLEELCSMSGNVTCLVQLLKQCHLVPQDLDIPNPMEASETVHLANGVYTSLQEL NSNFRQIIFPEALRCLMKGEYTLESMLHELDGLIEQTTDGVPLQTLVESLQAYLRNAAMGLEEETHAHYIDVARLLHAQYGELIQPRNGS VDETPKMSAGQMLLVAFDGMFAQVETAFSLLVEKLNKMEIPIAWRKIDIIREARSTQVNFFDDDNHRQVLEEIFFLKRLQTIKEFFRLCG TFSKTLSGSSSLEDQNTVNGPVQIVNVKTLFRNSCFSEDQMAKPIKAFTADFVRQLLIGLPNQALGLTLCSFISALGVDIIAQVEAKDFG AESKVSVDDLCKKAVEHNIQIGKFSQLVMNRATVLASSYDTAWKKHDLVRRLETSISSCKTSLQRVQLHIAMFQQWQHEDLLINRPQAMS VTPPPRSAILTSMKKKLHTLSQIETSIATVQEKLAALESSIEQRLKWAGGANPALAPVLQDFEATIAERRNLVLKESQRASQVTFLCSNI IHFESLRTRTAEALNLDAALFELIKRCQQMCSFASQFNSSVSELELRLLQRVDTGLEHPIGSSEWLLSAHKQLTQDMSTQRAIQTEKEQQ IETVCETIQNLVDNIKTVLTGHNRQLGDVKHLLKAMAKDEEAALADGEDVPYENSVRQFLGEYKSWQDNIQTVLFTLVQAMGQVRSQEHV EMLQEITPTLKELKTQSQSIYNNLVSFASPLVTDATNECSSPTSSATYQPSFAAAVRSNTGQKTQPDVMSQNARKLIQKNLATSADTPPS -------------------------------------------------------------- |
Multiple Sequence Alignment of All Fusion Protein Isoforms |
Top |
Kinase Fusion Protein Functional Features |
 Four levels of functional features of fusion genes Four levels of functional features of fusion genesGo to FGviewer search page for the most frequent breakpoint (https://ccsmweb.uth.edu/FGviewer/chr15:60646353/chr16:18883946) - FGviewer provides the online visualization of the retention search of the protein functional features across DNA, RNA, protein, and pathological levels. - How to search 1. Put your fusion gene symbol. 2. Press the tab key until there will be shown the breakpoint information filled. 4. Go down and press 'Search' tab twice. 4. Go down to have the hyperlink of the search result. 5. Click the hyperlink. 6. See the FGviewer result for your fusion gene. |
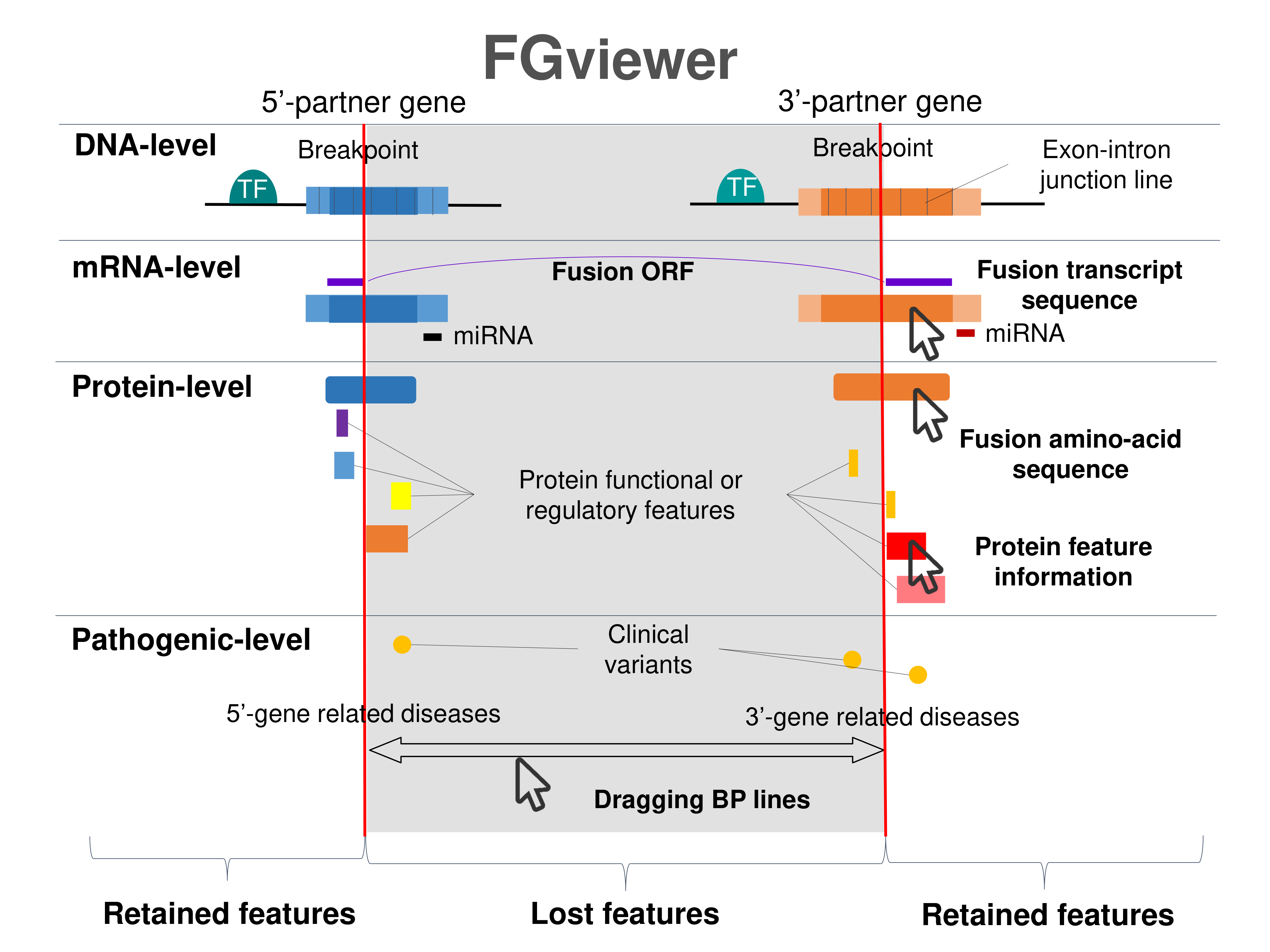 |
 Main function of each fusion partner protein. (from UniProt) Main function of each fusion partner protein. (from UniProt) |
| Hgene | Tgene |
| ANXA2 | SMG1 |
| FUNCTION: Calcium-regulated membrane-binding protein whose affinity for calcium is greatly enhanced by anionic phospholipids. It binds two calcium ions with high affinity. May be involved in heat-stress response. Inhibits PCSK9-enhanced LDLR degradation, probably reduces PCSK9 protein levels via a translational mechanism but also competes with LDLR for binding with PCSK9 (PubMed:18799458, PubMed:24808179, PubMed:22848640). {ECO:0000269|PubMed:18799458, ECO:0000269|PubMed:22848640, ECO:0000269|PubMed:24808179}.; FUNCTION: (Microbial infection) Binds M.pneumoniae CARDS toxin, probably serves as one receptor for this pathogen. When ANXA2 is down-regulated by siRNA, less toxin binds to human cells and less vacuolization (a symptom of M.pneumoniae infection) is seen. {ECO:0000269|PubMed:25139904}. | FUNCTION: Serine/threonine protein kinase involved in both mRNA surveillance and genotoxic stress response pathways. Recognizes the substrate consensus sequence [ST]-Q. Plays a central role in nonsense-mediated decay (NMD) of mRNAs containing premature stop codons by phosphorylating UPF1/RENT1. Recruited by release factors to stalled ribosomes together with SMG8 and SMG9 (forming the SMG1C protein kinase complex), and UPF1 to form the transient SURF (SMG1-UPF1-eRF1-eRF3) complex. In EJC-dependent NMD, the SURF complex associates with the exon junction complex (EJC) through UPF2 and allows the formation of an UPF1-UPF2-UPF3 surveillance complex which is believed to activate NMD. Also acts as a genotoxic stress-activated protein kinase that displays some functional overlap with ATM. Can phosphorylate p53/TP53 and is required for optimal p53/TP53 activation after cellular exposure to genotoxic stress. Its depletion leads to spontaneous DNA damage and increased sensitivity to ionizing radiation (IR). May activate PRKCI but not PRKCZ. {ECO:0000269|PubMed:11331269, ECO:0000269|PubMed:11544179, ECO:0000269|PubMed:15175154, ECO:0000269|PubMed:16452507}. |
 Retention analysis result of each fusion partner protein across 39 protein features of UniProt such as six molecule processing features, 13 region features, four site features, six amino acid modification features, two natural variation features, five experimental info features, and 3 secondary structure features. Here, because of limited space for viewing, we only show the protein feature retention information belong to the 13 regional features. All retention annotation result can be downloaded at * Minus value of BPloci means that the break pointn is located before the CDS. Retention analysis result of each fusion partner protein across 39 protein features of UniProt such as six molecule processing features, 13 region features, four site features, six amino acid modification features, two natural variation features, five experimental info features, and 3 secondary structure features. Here, because of limited space for viewing, we only show the protein feature retention information belong to the 13 regional features. All retention annotation result can be downloaded at * Minus value of BPloci means that the break pointn is located before the CDS. |
 - Retained domain in the 5'-partner of fusion protein (protein functional feature from UniProt). - Retained domain in the 5'-partner of fusion protein (protein functional feature from UniProt). |
| Partner | Hgeneene | Hbp | Tgeneene | Tbp | ENST | BPexon | TotalExon | Protein feature loci | BPloci | TotalLen | Feature | Note |
 - Retained domain in the 3'-partner of fusion protein (protein functional feature from UniProt). - Retained domain in the 3'-partner of fusion protein (protein functional feature from UniProt). |
| Partner | Hgeneene | Hbp | Tgeneene | Tbp | ENST | BPexon | TotalExon | Protein feature loci | BPloci | TotalLen | Feature | Note |
| Tgene | ANXA2 | 60646353 | SMG1 | 18883946 | ENST00000396024 | 12 | 63 | 1283_1866 | 630 | 3662 | Domain | Note=FAT;Ontology_term=ECO:0000255;evidence=ECO:0000255|PROSITE-ProRule:PRU00534 |
| Tgene | ANXA2 | 60646353 | SMG1 | 18883946 | ENST00000396024 | 12 | 63 | 3629_3661 | 630 | 3662 | Domain | Note=FATC;Ontology_term=ECO:0000255,ECO:0000255;evidence=ECO:0000255|PROSITE-ProRule:PRU00534,ECO:0000255|PROSITE-ProRule:PRU00535 |
| Tgene | ANXA2 | 60646353 | SMG1 | 18883946 | ENST00000396024 | 12 | 63 | 2124_2463 | 630 | 3662 | Domain | Note=PI3K/PI4K catalytic;Ontology_term=ECO:0000255;evidence=ECO:0000255|PROSITE-ProRule:PRU00269 |
Top |
Kinase Fusion Protein Structures |
 CIF files of the predicted kinase fusion proteins CIF files of the predicted kinase fusion proteins * Here we show the 3D structure of the fusion proteins using Mol*. AlphaFold produces a per-residue confidence score (pLDDT) between 0 and 100. Model confidence is shown from the pLDDT values per residue. pLDDT corresponds to the model’s prediction of its score on the local Distance Difference Test. It is a measure of local accuracy (from AlphfaFold website). To color code individual residues, we transformed individual PDB files into CIF format. |
| Kinase Fusion protein CIF link (fusion AA seq ID in KinaseFusionDB) | Henst | Tenst | Hgene | Hchr | Hbp | Tgene | Tchr | Tbp | AA seq | Len(AA seq) |
| PDB file >>>26_ANXA2_SMG1 | ENST00000396024 | ENST00000389467 | ANXA2 | chr15 | 60646353 | SMG1 | chr16 | 18883946 | MLCASFKMSTVHEILCKLSLEGDHSTPPSAYGSVKAYTNFDAERDALNIETAIKTKGVDEVTIVNILTNRSNAQRQDIAFAYQRRTKKEL ASALKSALSGHLETVILGLLKTPAQYDASELKASMKGLGTDEDSLIEIICSRTNQELQEINRVYKEMYKTDLEKDIISDTSGDFRKLMVA LAKGRRAEDGSVIDYELIDQDARMWALSPTVFALLSKNLMIVHSDLAVHFPAIQYAVLYTLYSHCTRHDHFISSSLSSSSPSLFDGAVIS TVTTATKKHFSIILNLLGILLKKDNLNQDTRKLLMTWALEAAVLMKKSETYAPLFSLPSFHKFCKGLLANTLVEDVNICLQACSSLHALS SSLPDDLLQRCVDVCRVQLVHSGTRIRQAFGKLLKSIPLDVVLSNNNHTEIQEISLALRSHMSKAPSNTFHPQDFSDVISFILYGNSHRT GKDNWLERLFYSCQRLDKRDQSTIPRNLLKTDAVLWQWAIWEAAQFTVLSKLRTPLGRAQDTFQTIEGIIRSLAAHTLNPDQDVSQWTTA DNDEGHGNNQLRLVLLLQYLENLEKLMYNAYEGCANALTSPPKVIRTFFYTNRQTCQDWLTRIRLSIMRVGLLAGQPAVTVRHGFDLLTE MKTTSLSQGNELEVTIMMVVEALCELHCPEAIQGIAVWSSSIVGKNLLWINSVAQQAEGRFEKASVEYQEHLCAMTGVDCCISSFDKSVL TLANAGRNSASPKHSLNGESRKTVLSKPTDSSPEVINYLGNKACECYISIADWAAVQEWQNAIHDLKKSTSSTSLNLKADFNYIKSLSSF ESGKFVECTEQLELLPGENINLLAGGSKEKIDMKKLLPNMLSPDPRELQKSIEVQLLRSSVCLATALNPIEQDQKWQSITENVVKYLKQT SRIAIGPLRLSTLTVSQSLPVLSTLQLYCSSALENTVSNRLSTEDCLIPLFSEALRSCKQHDVRPWMQALRYTMYQNQLLEKIKEQTVPI RSHLMELGLTAAKFARKRGNVSLATRLLAQCSEVQLGKTTTAQDLVQHFKKLSTQGQVDEKWGPELDIEKTKLLYTAGQSTHAMEMLSSC AISFCKSVKAEYAVAKSILTLAKWIQAEWKEISGQLKQVYRAQHQQNFTGLSTLSKNILTLIELPSVNTMEEEYPRIESESTVHIGVGEP DFILGQLYHLSSVQAPEVAKSWAALASWAYRWGRKVVDNASQGEGVRLLPREKSEVQNLLPDTITEEEKERIYGILGQAVCRPAGIQDED ITLQITESEDNEEDDMVDVIWRQLISSCPWLSELDESATEGVIKVWRKVVDRIFSLYKLSCSAYFTFLKLNAGQIPLDEDDPRLHLSHRV EQSTDDMIVMATLRLLRLLVKHAGELRQYLEHGLETTPTAPWRGIIPQLFSRLNHPEVYVRQSICNLLCRVAQDSPHLILYPAIVGTISL SSESQASGNKFSTAIPTLLGNIQGEELLVSECEGGSPPASQDSNKDEPKSGLNEDQAMMQDCYSKIVDKLSSANPTMVLQVQMLVAELRR VTVLWDELWLGVLLQQHMYVLRRIQQLEDEVKRVQNNNTLRKEEKIAIMREKHTALMKPIVFALEHVRSITAAPAETPHEKWFQDNYGDA IENALEKLKTPLNPAKPGSSWIPFKEIMLSLQQRAQKRASYILRLEEISPWLAAMTNTEIALPGEVSARDTVTIHSVGGTITILPTKTKP KKLLFLGSDGKSYPYLFKGLEDLHLDERIMQFLSIVNTMFATINRQETPRFHARHYSVTPLGTRSGLIQWVDGATPLFGLYKRWQQREAA LQAQKAQDSYQTPQNPGIVPRPSELYYSKIGPALKTVGLSLDVSRRDWPLHVMKAVLEELMEATPPNLLAKELWSSCTTPDEWWRVTQSY ARSTAVMSMVGYIIGLGDRHLDNVLIDMTTGEVVHIDYNVCFEKGKSLRVPEKVPFRMTQNIETALGVTGVEGVFRLSCEQVLHIMRRGR ETLLTLLEAFVYDPLVDWTAGGEAGFAGAVYGGGGQQAESKQSKREMEREITRSLFSSRVAEIKVNWFKNRDEMLVVLPKLDGSLDEYLS LQEQLTDVEKLQGKLLEEIEFLEGAEGVDHPSHTLQHRYSEHTQLQTQQRAVQEAIQVKLNEFEQWITHYQAAFNNLEATQLASLLQEIS TQMDLGPPSYVPATAFLQNAGQAHLISQCEQLEGEVGALLQQRRSVLRGCLEQLHHYATVALQYPKAIFQKHRIEQWKTWMEELICNTTV ERCQELYRKYEMQYAPQPPPTVCQFITATEMTLQRYAADINSRLIRQVERLKQEAVTVPVCEDQLKEIERCIKVFLHENGEEGSLSLASV IISALCTLTRRNLMMEGAASSAGEQLVDLTSRDGAWFLEELCSMSGNVTCLVQLLKQCHLVPQDLDIPNPMEASETVHLANGVYTSLQEL NSNFRQIIFPEALRCLMKGEYTLESMLHELDGLIEQTTDGVPLQTLVESLQAYLRNAAMGLEEETHAHYIDVARLLHAQYGELIQPRNGS VDETPKMSAGQMLLVAFDGMFAQVETAFSLLVEKLNKMEIPIAWRKIDIIREARSTQVNFFDDDNHRQVLEEIFFLKRLQTIKEFFRLCG TFSKTLSGSSSLEDQNTVNGPVQIVNVKTLFRNSCFSEDQMAKPIKAFTADFVRQLLIGLPNQALGLTLCSFISALGVDIIAQVEAKDFG AESKVSVDDLCKKAVEHNIQIGKFSQLVMNRATVLASSYDTAWKKHDLVRRLETSISSCKTSLQRVQLHIAMFQQWQHEDLLINRPQAMS VTPPPRSAILTSMKKKLHTLSQIETSIATVQEKLAALESSIEQRLKWAGGANPALAPVLQDFEATIAERRNLVLKESQRASQVTFLCSNI IHFESLRTRTAEALNLDAALFELIKRCQQMCSFASQFNSSVSELELRLLQRVDTGLEHPIGSSEWLLSAHKQLTQDMSTQRAIQTEKEQQ IETVCETIQNLVDNIKTVLTGHNRQLGDVKHLLKAMAKDEEAALADGEDVPYENSVRQFLGEYKSWQDNIQTVLFTLVQAMGQVRSQEHV EMLQEITPTLKELKTQSQSIYNNLVSFASPLVTDATNECSSPTSSATYQPSFAAAVRSNTGQKTQPDVMSQNARKLIQKNLATSADTPPS | 3235 |
| 3D view using mol* of 26_ANXA2_SMG1 | ||||||||||
| PDB file >>>TKFP_41_ANXA2_SMG1 | ENST00000396024 | ENST00000389467 | ANXA2 | chr15 | 60646353 | SMG1 | chr16 | 18883946 | MLCASFKMSTVHEILCKLSLEGDHSTPPSAYGSVKAYTNFDAERDALNIETAIKTKGVDEVTIVNILTNRSNAQRQDIAFAYQRRTKKEL ASALKSALSGHLETVILGLLKTPAQYDASELKASMKGLGTDEDSLIEIICSRTNQELQEINRVYKEMYKTDLEKDIISDTSGDFRKLMVA LAKGRRAEDGSVIDYELIDQDARMWALSPTVFALLSKNLMIVHSDLAVHFPAIQYAVLYTLYSHCTRHDHFISSSLSSSSPSLFDGAVIS TVTTATKKHFSIILNLLGILLKKDNLNQDTRKLLMTWALEAAVLMKKSETYAPLFSLPSFHKFCKGLLANTLVEDVNICLQACSSLHALS SSLPDDLLQRCVDVCRVQLVHSGTRIRQAFGKLLKSIPLDVVLSNNNHTEIQEISLALRSHMSKAPSNTFHPQDFSDVISFILYGNSHRT GKDNWLERLFYSCQRLDKRDQSTIPRNLLKTDAVLWQWAIWEAAQFTVLSKLRTPLGRAQDTFQTIEGIIRSLAAHTLNPDQDVSQWTTA DNDEGHGNNQLRLVLLLQYLENLEKLMYNAYEGCANALTSPPKVIRTFFYTNRQTCQDWLTRIRLSIMRVGLLAGQPAVTVRHGFDLLTE MKTTSLSQGNELEVTIMMVVEALCELHCPEAIQGIAVWSSSIVGKNLLWINSVAQQAEGRFEKASVEYQEHLCAMTGVDCCISSFDKSVL TLANAGRNSASPKHSLNGESRKTVLSKPTDSSPEVINYLGNKACECYISIADWAAVQEWQNAIHDLKKSTSSTSLNLKADFNYIKSLSSF ESGKFVECTEQLELLPGENINLLAGGSKEKIDMKKLLPNMLSPDPRELQKSIEVQLLRSSVCLATALNPIEQDQKWQSITENVVKYLKQT SRIAIGPLRLSTLTVSQSLPVLSTLQLYCSSALENTVSNRLSTEDCLIPLFSEALRSCKQHDVRPWMQALRYTMYQNQLLEKIKEQTVPI RSHLMELGLTAAKFARKRGNVSLATRLLAQCSEVQLGKTTTAQDLVQHFKKLSTQGQVDEKWGPELDIEKTKLLYTAGQSTHAMEMLSSC AISFCKSVKAEYAVAKSILTLAKWIQAEWKEISGQLKQVYRAQHQQNFTGLSTLSKNILTLIELPSVNTMEEEYPRIESESTVHIGVGEP DFILGQLYHLSSVQAPEVAKSWAALASWAYRWGRKVVDNASQGEGVRLLPREKSEVQNLLPDTITEEEKERIYGILGQAVCRPAGIQDED ITLQITESEDNEEDDMVDVIWRQLISSCPWLSELDESATEGVIKVWRKVVDRIFSLYKLSCSAYFTFLKLNAGQIPLDEDDPRLHLSHRV EQSTDDMIVMATLRLLRLLVKHAGELRQYLEHGLETTPTAPWRGIIPQLFSRLNHPEVYVRQSICNLLCRVAQDSPHLILYPAIVGTISL SSESQASGNKFSTAIPTLLGNIQGEELLVSECEGGSPPASQDSNKDEPKSGLNEDQAMMQDCYSKIVDKLSSANPTMVLQVQMLVAELRR VTVLWDELWLGVLLQQHMYVLRRIQQLEDEVKRVQNNNTLRKEEKIAIMREKHTALMKPIVFALEHVRSITAAPAETPHEKWFQDNYGDA IENALEKLKTPLNPAKPGSSWIPFKEIMLSLQQRAQKRASYILRLEEISPWLAAMTNTEIALPGEVSARDTVTIHSVGGTITILPTKTKP KKLLFLGSDGKSYPYLFKGLEDLHLDERIMQFLSIVNTMFATINRQETPRFHARHYSVTPLGTRSGLIQWVDGATPLFGLYKRWQQREAA LQAQKAQDSYQTPQNPGIVPRPSELYYSKIGPALKTVGLSLDVSRRDWPLHVMKAVLEELMEATPPNLLAKELWSSCTTPDEWWRVTQSY ARSTAVMSMVGYIIGLGDRHLDNVLIDMTTGEVVHIDYNVCFEKGKSLRVPEKVPFRMTQNIETALGVTGVEGVFRLSCEQVLHIMRRGR ETLLTLLEAFVYDPLVDWTAGGEAGFAGAVYGGGGQQAESKQSKREMEREITRSLFSSRVAEIKVNWFKNRDEMLVVLPKLDGSLDEYLS LQEQLTDVEKLQGKLLEEIEFLEGAEGVDHPSHTLQHRYSEHTQLQTQQRAVQEAIQVKLNEFEQWITHYQAAFNNLEATQLASLLQEIS TQMDLGPPSYVPATAFLQNAGQAHLISQCEQLEGEVGALLQQRRSVLRGCLEQLHHYATVALQYPKAIFQKHRIEQWKTWMEELICNTTV ERCQELYRKYEMQYAPQPPPTVCQFITATEMTLQRYAADINSRLIRQVERLKQEAVTVPVCEDQLKEIERCIKVFLHENGEEGSLSLASV IISALCTLTRRNLMMEGAASSAGEQLVDLTSRDGAWFLEELCSMSGNVTCLVQLLKQCHLVPQDLDIPNPMEASETVHLANGVYTSLQEL NSNFRQIIFPEALRCLMKGEYTLESMLHELDGLIEQTTDGVPLQTLVESLQAYLRNAAMGLEEETHAHYIDVARLLHAQYGELIQPRNGS VDETPKMSAGQMLLVAFDGMFAQVETAFSLLVEKLNKMEIPIAWRKIDIIREARSTQVNFFDDDNHRQVLEEIFFLKRLQTIKEFFRLCG TFSKTLSGSSSLEDQNTVNGPVQIVNVKTLFRNSCFSEDQMAKPIKAFTADFVRQLLIGLPNQALGLTLCSFISALGVDIIAQVEAKDFG AESKVSVDDLCKKAVEHNIQIGKFSQLVMNRATVLASSYDTAWKKHDLVRRLETSISSCKTSLQRVQLHIAMFQQWQHEDLLINRPQAMS VTPPPRSAILTSMKKKLHTLSQIETSIATVQEKLAALESSIEQRLKWAGGANPALAPVLQDFEATIAERRNLVLKESQRASQVTFLCSNI IHFESLRTRTAEALNLDAALFELIKRCQQMCSFASQFNSSVSELELRLLQRVDTGLEHPIGSSEWLLSAHKQLTQDMSTQRAIQTEKEQQ IETVCETIQNLVDNIKTVLTGHNRQLGDVKHLLKAMAKDEEAALADGEDVPYENSVRQFLGEYKSWQDNIQTVLFTLVQAMGQVRSQEHV EMLQEITPTLKELKTQSQSIYNNLVSFASPLVTDATNECSSPTSSATYQPSFAAAVRSNTGQKTQPDVMSQNARKLIQKNLATSADTPPS | 3235_ANXA2_SMG1 |
Top |
Comparison of Fusion Protein Isoforms |
 Superimpose the 3D Structures Among All Fusion Protein Isoforms Superimpose the 3D Structures Among All Fusion Protein Isoforms * Download the pdb file and open it from the molstar online viewer. |
 Comparison of the Secondary Structures of Fusion Protein Isoforms Comparison of the Secondary Structures of Fusion Protein Isoforms |
Top |
Comparison of Fusion Protein Sequences/Structures with Known Sequences/Structures from PDB |
Top |
pLDDT score distribution |
 pLDDT score distribution of the predicted fusion protein structures from AlphaFold2 pLDDT score distribution of the predicted fusion protein structures from AlphaFold2* AlphaFold produces a per-residue confidence score (pLDDT) between 0 and 100. * The blue color at the bottom marks the best active site residues. |
Top |
Potential Active Site Information |
 The potential binding sites of these fusion proteins were identified using SiteMap, a module of the Schrodinger suite. The potential binding sites of these fusion proteins were identified using SiteMap, a module of the Schrodinger suite. |
| Kinase Fusion AA seq ID in KinaseFusionDB | Site score | Size | Dscore | Volume | Exposure | Enclosure | Contact | Phobic | Philic | Balance | Don/Acc | Residues |
Top |
Ramachandran Plot of Kinase Fusion Protein Structure |
 Ramachandran plot of the torsional angles - phi (φ)and psi (ψ) - of the residues (amino acids) contained in this fusion protein peptide. Ramachandran plot of the torsional angles - phi (φ)and psi (ψ) - of the residues (amino acids) contained in this fusion protein peptide. |
Top |
Virtual Screening Results |
 Distribution of the average docking score across all approved kinase inhibitors. Distribution of the average docking score across all approved kinase inhibitors.Distribution of the number of occurrence across all approved kinase inhibitors. |
| 5'-kinase fusion protein case |
| 3'-kinase fusion protein case |
Top |
 Drug information from DrugBank of the top 20 interacting small molecules. Drug information from DrugBank of the top 20 interacting small molecules.* The detailed information of individual kinase inhibitors are available in the download page. |
| Fusion gene name info | Drug | Docking score | Glide g score | Glide energy |
Top |
Kinase-Substrate Information of ANXA2_SMG1 |
 Phosphorylation target of the kinase Phosphorylation target of the kinase(phosphosite, 03-17-2024) |
| Kinase | Kinase UniProt Acc | Kinase species | Substrate | Substrate UniProt Acc | Substrate phosphorylated residues | Substrate phosphorylated sites (+/-7AA) | Domain |
| SMG1 | Q96Q15 | human | UPF1 | Q92900 | T28 | AELLGADtQGsEFEF | |
| SMG1 | Q96Q15 | human | UPF1 | Q92900 | S1089 | GLsQPELsQDSYLGD | |
| SMG1 | Q96Q15 | human | UPF1 | Q92900 | S1127 | HGGVTGLsQy_____ | |
| SMG1 | Q96Q15 | human | UPF1 | Q92900 | S1107 | sQIDVALsQDstyQG | |
| SMG1 | Q96Q15 | human | UPF1 | Q92900 | S1084 | QMSQPGLsQPELsQD | |
| SMG1 | Q96Q15 | human | TP53 | P04637 | S15 | PsVEPPLsQEtFsDL | P53_TAD |
 Biological Network Integration of This Kinase and Substrates Biological Network Integration of This Kinase and Substrates (GeneMANIA website) |
 Enriched GO biological processes of the phosphorylation target genes of the kinase Enriched GO biological processes of the phosphorylation target genes of the kinase |
| Kinase | GOID | GO term | P.adjust |
| SMG1 | ID | Description | 0.00e+00 |
| SMG1 | GO:0071216 | cellular response to biotic stimulus | 1.83e-02 |
| SMG1 | GO:0006260 | DNA replication | 1.83e-02 |
| SMG1 | GO:0006913 | nucleocytoplasmic transport | 1.83e-02 |
| SMG1 | GO:0051169 | nuclear transport | 1.83e-02 |
| SMG1 | GO:0022411 | cellular component disassembly | 1.83e-02 |
| SMG1 | GO:0061418 | regulation of transcription from RNA polymerase II promoter in response to hypoxia | 1.83e-02 |
| SMG1 | GO:1900543 | negative regulation of purine nucleotide metabolic process | 1.83e-02 |
| SMG1 | GO:1903799 | negative regulation of miRNA processing | 1.83e-02 |
| SMG1 | GO:0006983 | ER overload response | 1.83e-02 |
| SMG1 | GO:0007406 | negative regulation of neuroblast proliferation | 1.83e-02 |
| SMG1 | GO:0045980 | negative regulation of nucleotide metabolic process | 1.83e-02 |
| SMG1 | GO:0051095 | regulation of helicase activity | 1.83e-02 |
| SMG1 | GO:0062100 | positive regulation of programmed necrotic cell death | 1.83e-02 |
| SMG1 | GO:0071044 | histone mRNA catabolic process | 1.83e-02 |
| SMG1 | GO:1990440 | positive regulation of transcription from RNA polymerase II promoter in response to endoplasmic reticulum stress | 1.83e-02 |
| SMG1 | GO:0060965 | negative regulation of miRNA-mediated gene silencing | 1.83e-02 |
| SMG1 | GO:0071236 | cellular response to antibiotic | 1.83e-02 |
| SMG1 | GO:0060149 | negative regulation of post-transcriptional gene silencing | 1.83e-02 |
| SMG1 | GO:0060967 | negative regulation of gene silencing by regulatory ncRNA | 1.83e-02 |
| SMG1 | GO:0070234 | positive regulation of T cell apoptotic process | 1.83e-02 |
| SMG1 | GO:1900369 | negative regulation of post-transcriptional gene silencing by regulatory ncRNA | 1.83e-02 |
| SMG1 | GO:0010225 | response to UV-C | 1.83e-02 |
| SMG1 | GO:0070243 | regulation of thymocyte apoptotic process | 1.83e-02 |
| SMG1 | GO:0034349 | glial cell apoptotic process | 1.83e-02 |
| SMG1 | GO:0070230 | positive regulation of lymphocyte apoptotic process | 1.83e-02 |
| SMG1 | GO:1901524 | regulation of mitophagy | 1.83e-02 |
| SMG1 | GO:1903798 | regulation of miRNA processing | 1.83e-02 |
| SMG1 | GO:0006415 | translational termination | 1.83e-02 |
| SMG1 | GO:0032780 | negative regulation of ATP-dependent activity | 1.83e-02 |
| SMG1 | GO:0060253 | negative regulation of glial cell proliferation | 1.83e-02 |
| SMG1 | GO:0070920 | regulation of regulatory ncRNA processing | 1.83e-02 |
| SMG1 | GO:0090399 | replicative senescence | 1.83e-02 |
| SMG1 | GO:1901522 | positive regulation of transcription from RNA polymerase II promoter involved in cellular response to chemical stimulus | 1.83e-02 |
| SMG1 | GO:0006089 | lactate metabolic process | 1.83e-02 |
| SMG1 | GO:0006977 | DNA damage respons | 1.91e-03 |
| SMG1 | GO:0010663 | positive regulation of striated muscle cell apoptotic process | 1.83e-02 |
| SMG1 | GO:0010666 | positive regulation of cardiac muscle cell apoptotic process | 1.83e-02 |
| SMG1 | GO:0042772 | DNA damage respons | 2.01e-03 |
| SMG1 | GO:0070314 | G1 to G0 transition | 1.83e-02 |
| SMG1 | GO:2000774 | positive regulation of cellular senescence | 1.83e-02 |
| SMG1 | GO:0006098 | pentose-phosphate shunt | 1.83e-02 |
| SMG1 | GO:0008334 | histone mRNA metabolic process | 1.83e-02 |
| SMG1 | GO:0070242 | thymocyte apoptotic process | 1.83e-02 |
| SMG1 | GO:2000269 | regulation of fibroblast apoptotic process | 1.83e-02 |
| SMG1 | GO:0032042 | mitochondrial DNA metabolic process | 1.83e-02 |
| SMG1 | GO:0006740 | NADPH regeneration | 1.83e-02 |
| SMG1 | GO:0009651 | response to salt stress | 1.83e-02 |
| SMG1 | GO:0008156 | negative regulation of DNA replication | 1.83e-02 |
| SMG1 | GO:0043153 | entrainment of circadian clock by photoperiod | 1.83e-02 |
Top |
Related Drugs to ANXA2_SMG1 |
 Drugs used for this fusion-positive patient. Drugs used for this fusion-positive patient. (Manual curation of PubMed, 04-30-2022 + MyCancerGenome) |
| Hgene | Tgene | Drug | Source | PMID |
 Distribution of the number of studies mentioning ANXA2-SMG1 and kinase inhibitors the PubMed Abstract (04-01-2024) Distribution of the number of studies mentioning ANXA2-SMG1 and kinase inhibitors the PubMed Abstract (04-01-2024) |
| Fusion gene - drug pair 1 | Fusion gene - drug pair 2 | PMID | Publication date | DOI | Study title |
Top |
Related Diseases to ANXA2_SMG1 |
 Diseases that have this fusion gene. Diseases that have this fusion gene. (Manual curation of PubMed, 04-30-2022 + MyCancerGenome) |
| Hgene | Tgene | Disease | Source | PMID |
 Related diseases from the literature mentioned this fusion gene and drug. Related diseases from the literature mentioned this fusion gene and drug. (PubMed, 04-01-2024) |
| MeSH ID | MeSH term |
 Diseases associated with fusion partners. Diseases associated with fusion partners. (DisGeNet 4.0) |
| Partner | Gene | Disease ID | Disease name | # pubmeds | Source |
Top |
Clinical Trials of the Found Drugs/Small Molecules |
 Statistics of the Clinical Trials of the Found Kinase Inibitors from clinicaltrials.gov (06-17-2024) Statistics of the Clinical Trials of the Found Kinase Inibitors from clinicaltrials.gov (06-17-2024) |
 Clinical Trials from clinicaltrials.gov (06-17-2024) Clinical Trials from clinicaltrials.gov (06-17-2024) |
| Fusion Gene | Kinase Inhibitor | NCT ID | Study Status | Phases | Disease | # Enrolment | Date |

