| UTHEALTH HOME ABOUT SBMI A-Z WEBMAIL INSIDE THE UNIVERSITY |

|
|||||||
|
Kinase Fusion Gene:PAK1_PAK2 |
Kinase Fusion Protein Summary |
 Kinase Fusion gene summary Kinase Fusion gene summary |
| Kinase Fusion partner gene information | Kinase Fusion gene name: PAK1_PAK2 | KinaseFusionDB ID: KFG4422 | FusionGDB2.0 ID: KFG4422 | Hgene | Tgene | Gene symbol | PAK1 | PAK2 | Gene ID | 5585 | 5586 | |
| Gene name | protein kinase N1 | protein kinase N2 | ||||||||||
| Synonyms | DBK|PAK-1|PAK1|PKN|PKN-ALPHA|PRK1|PRKCL1 | PAK2|PRK2|PRKCL2|PRO2042|Pak-2|STK7 | ||||||||||
| Cytomap | 19p13.12 | 1p22.2 | ||||||||||
| Type of gene | protein-coding | protein-coding | ||||||||||
| Description | serine/threonine-protein kinase N1protease-activated kinase 1protein kinase C-like 1protein kinase C-like PKNprotein kinase C-related kinase 1protein kinase PKN-alphaserine-threonine kinase Nserine/threonine protein kinase N | serine/threonine-protein kinase N2PKN gammacardiolipin-activated protein kinase Pak2protein kinase C-like 2protein-kinase C-related kinase 2 | ||||||||||
| Modification date | 20240305 | 20240323 | ||||||||||
| UniProtAcc | Q16512 | Q13177 | ||||||||||
| Ensembl transtripts involved in fusion gene | ENST ids | ENST00000356341, ENST00000530617, ENST00000278568, ENST00000528203, ENST00000525542, | ENST00000327134, | |||||||||
| Context (manual curation of fusion genes in KinaseFusionDB) | PubMed: PAK1 [Title/Abstract] AND PAK2 [Title/Abstract] AND fusion [Title/Abstract] | |||||||||||
| Most frequent breakpoint (based on all fusion genes of FusionGDB 2.0) | ||||||||||||
 Gene ontology of each fusion partner gene with evidence of Inferred from Direct Assay (IDA) from Entrez Gene ontology of each fusion partner gene with evidence of Inferred from Direct Assay (IDA) from Entrez |
| Partner | Gene | GO ID | GO term | PubMed ID |
| Hgene | PAK1 | GO:0006357 | regulation of transcription by RNA polymerase II | 12514133 |
| Hgene | PAK1 | GO:0006468 | protein phosphorylation | 17332740 |
| Hgene | PAK1 | GO:0043687 | post-translational protein modification | 18066052 |
| Tgene | PAK2 | GO:0006468 | protein phosphorylation | 17332740 |
| Tgene | PAK2 | GO:0010631 | epithelial cell migration | 21754995 |
 Kinase Fusion gene breakpoints across PAK1 (5'-gene) Kinase Fusion gene breakpoints across PAK1 (5'-gene)* Click on the image to open the UCSC genome browser with custom track showing this image in a new window. |
 Kinase Fusion gene breakpoints across PAK2 (3'-gene) Kinase Fusion gene breakpoints across PAK2 (3'-gene)* Click on the image to open the UCSC genome browser with custom track showing this image in a new window. |
Top |
Kinase Fusion Gene Sample Information |
 Kinase Fusion gene information. Kinase Fusion gene information. |
 Kinase Fusion gene information from four resources (ChiTars 5.0, ChimerDB 4.0, COSMIC, and CCLE) Kinase Fusion gene information from four resources (ChiTars 5.0, ChimerDB 4.0, COSMIC, and CCLE)* All genome coordinats were lifted-over on hg19. * Click on the break point to see the gene structure around the break point region using the UCSC Genome Browser. |
| Source | Sample | Hgene | Hchr | Hbp | Tgene | Tchr | Tbp |
| CCLE | MDA-MB-134-VI | PAK1 | chr11 | 77047130 | PAK2 | chr3 | 196547409 |
Top |
Kinase Fusion ORF Analysis |
 Kinase Fusion information from ORFfinder translation from full-length transcript sequence from KinaseFusionDB. Kinase Fusion information from ORFfinder translation from full-length transcript sequence from KinaseFusionDB. |
| Henst | Tenst | Hgene | Hchr | Hbp | Tgene | Tchr | Tbp | Seq length (transcript) | Seq length (amino acids) |
| ENST00000530617 | ENST00000327134 | PAK1 | chr11 | 77047130 | PAK2 | chr3 | 196547409 | 6221 | 485 |
Top |
Kinase Fusion Amino Acid Sequences |
 For individual full-length fusion transcript sequence from KinaseFusionDB, we ran ORFfinder and chose the longest ORF among the all predicted ones. For individual full-length fusion transcript sequence from KinaseFusionDB, we ran ORFfinder and chose the longest ORF among the all predicted ones. |
| >Henst_Tenst_Hgene_Hchr_Hbp_Tgene_Tchr_Tbp_length(fusion AA)_AAseq >ENST00000530617_ENST00000327134_PAK1_chr11_77047130_PAK2_chr3_196547409_length(amino acids)=485 MLLVVTMSNNGLDIQDKPPAPPMRNTSTMIGAGSKDAGTLNHGSKPLPPNPEEKKKKDRFYRSILPGDKTNKKKEKERPEISLPSDFEHT IHVGFDAVTGEFTGMPEQWARLLQTSNITKSEQKKNPQAVLDVLEFYNSKKTSNSQKYMSFTDKSAEDYNSSNALNVKAVSETPAVPPVS EDEDDDDDDATPPPVIAPRPEHTKSVYTRSVIEPLPVTPTRDVATSPISPTENNTTPPDALTRNTEKQKKKPKMSDEEILEKLRSIVSVG DPKKKYTRFEKIGQGASGTVYTAMDVATGQEVAIKQMNLQQQPKKELIINEILVMRENKNPNIVNYLDSYLVGDELWVVMEYLAGGSLTD VVTETCMDEGQIAAVCRECLQALEFLHSNQVIHRDIKSDNILLGMDGSVKLTDFGFCAQITPEQSKRSTMVGTPYWMAPEVVTRKAYGPK -------------------------------------------------------------- |
Multiple Sequence Alignment of All Fusion Protein Isoforms |
Top |
Kinase Fusion Protein Functional Features |
 Four levels of functional features of fusion genes Four levels of functional features of fusion genesGo to FGviewer search page for the most frequent breakpoint (https://ccsmweb.uth.edu/FGviewer/chr11:/chr3:) - FGviewer provides the online visualization of the retention search of the protein functional features across DNA, RNA, protein, and pathological levels. - How to search 1. Put your fusion gene symbol. 2. Press the tab key until there will be shown the breakpoint information filled. 4. Go down and press 'Search' tab twice. 4. Go down to have the hyperlink of the search result. 5. Click the hyperlink. 6. See the FGviewer result for your fusion gene. |
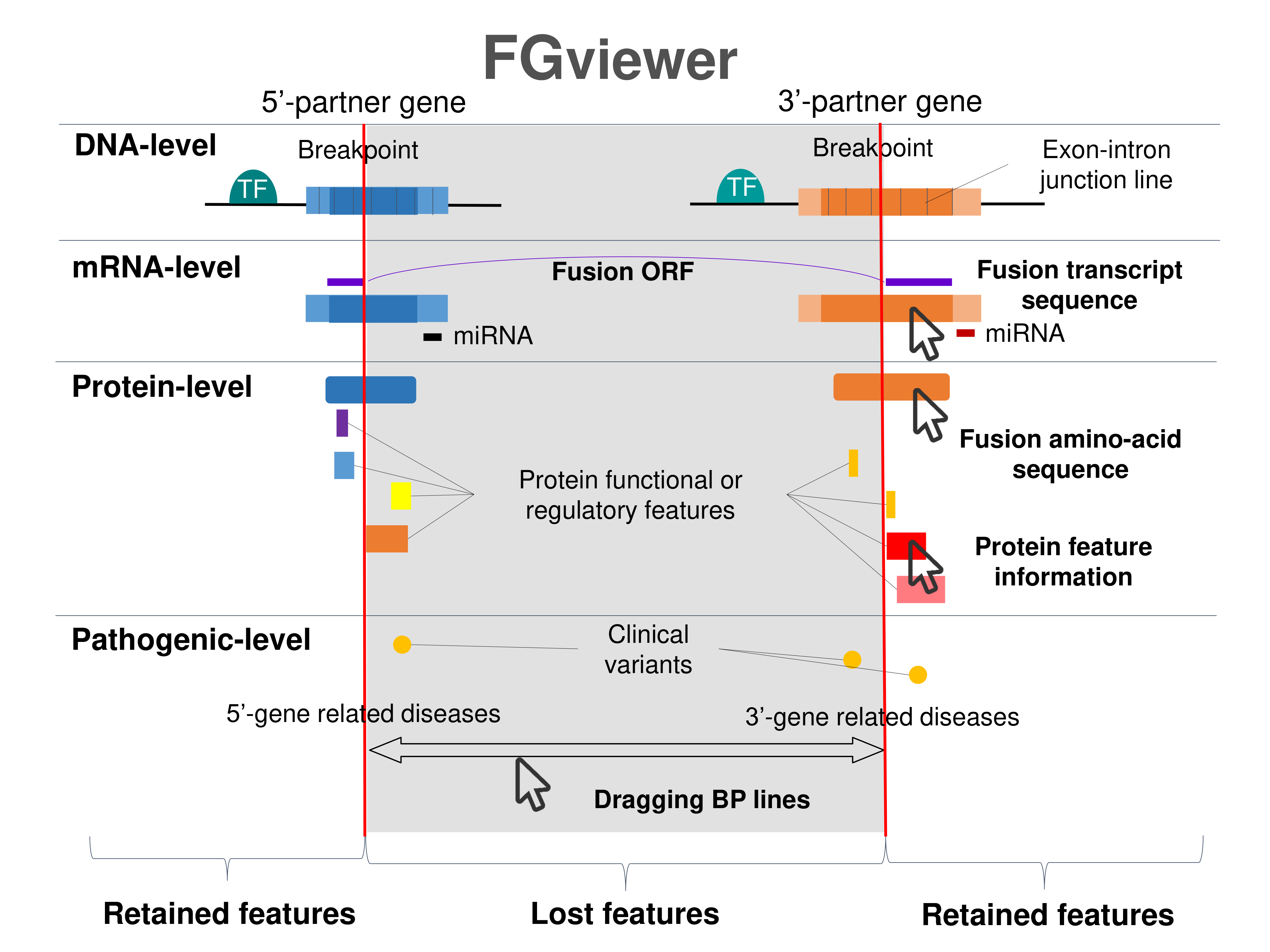 |
 Main function of each fusion partner protein. (from UniProt) Main function of each fusion partner protein. (from UniProt) |
| Hgene | Tgene |
| PAK1 | PAK2 |
| FUNCTION: PKC-related serine/threonine-protein kinase involved in various processes such as regulation of the intermediate filaments of the actin cytoskeleton, cell migration, tumor cell invasion and transcription regulation. Part of a signaling cascade that begins with the activation of the adrenergic receptor ADRA1B and leads to the activation of MAPK14. Regulates the cytoskeletal network by phosphorylating proteins such as VIM and neurofilament proteins NEFH, NEFL and NEFM, leading to inhibit their polymerization. Phosphorylates 'Ser-575', 'Ser-637' and 'Ser-669' of MAPT/Tau, lowering its ability to bind to microtubules, resulting in disruption of tubulin assembly. Acts as a key coactivator of androgen receptor (AR)-dependent transcription, by being recruited to AR target genes and specifically mediating phosphorylation of 'Thr-11' of histone H3 (H3T11ph), a specific tag for epigenetic transcriptional activation that promotes demethylation of histone H3 'Lys-9' (H3K9me) by KDM4C/JMJD2C. Phosphorylates HDAC5, HDAC7 and HDAC9, leading to impair their import in the nucleus. Phosphorylates 'Thr-38' of PPP1R14A, 'Ser-159', 'Ser-163' and 'Ser-170' of MARCKS, and GFAP. Able to phosphorylate RPS6 in vitro. {ECO:0000269|PubMed:11104762, ECO:0000269|PubMed:12514133, ECO:0000269|PubMed:17332740, ECO:0000269|PubMed:18066052, ECO:0000269|PubMed:20188095, ECO:0000269|PubMed:21224381, ECO:0000269|PubMed:21754995, ECO:0000269|PubMed:24248594, ECO:0000269|PubMed:8557118, ECO:0000269|PubMed:8621664, ECO:0000269|PubMed:9175763}. | FUNCTION: Serine/threonine protein kinase that plays a role in a variety of different signaling pathways including cytoskeleton regulation, cell motility, cell cycle progression, apoptosis or proliferation (PubMed:7744004, PubMed:19273597, PubMed:19923322, PubMed:9171063, PubMed:12853446, PubMed:16617111, PubMed:33693784). Acts as a downstream effector of the small GTPases CDC42 and RAC1 (PubMed:7744004). Activation by the binding of active CDC42 and RAC1 results in a conformational change and a subsequent autophosphorylation on several serine and/or threonine residues (PubMed:7744004). Full-length PAK2 stimulates cell survival and cell growth (PubMed:7744004). Phosphorylates MAPK4 and MAPK6 and activates the downstream target MAPKAPK5, a regulator of F-actin polymerization and cell migration (PubMed:21317288). Phosphorylates JUN and plays an important role in EGF-induced cell proliferation (PubMed:21177766). Phosphorylates many other substrates including histone H4 to promote assembly of H3.3 and H4 into nucleosomes, BAD, ribosomal protein S6, or MBP (PubMed:21724829). Phosphorylates CASP7, thereby preventing its activity (PubMed:21555521, PubMed:27889207). Additionally, associates with ARHGEF7 and GIT1 to perform kinase-independent functions such as spindle orientation control during mitosis (PubMed:19273597, PubMed:19923322). On the other hand, apoptotic stimuli such as DNA damage lead to caspase-mediated cleavage of PAK2, generating PAK-2p34, an active p34 fragment that translocates to the nucleus and promotes cellular apoptosis involving the JNK signaling pathway (PubMed:9171063, PubMed:12853446, PubMed:16617111). Caspase-activated PAK2 phosphorylates MKNK1 and reduces cellular translation (PubMed:15234964). {ECO:0000269|PubMed:12853446, ECO:0000269|PubMed:15234964, ECO:0000269|PubMed:16617111, ECO:0000269|PubMed:19273597, ECO:0000269|PubMed:19923322, ECO:0000269|PubMed:21177766, ECO:0000269|PubMed:21317288, ECO:0000269|PubMed:21555521, ECO:0000269|PubMed:21724829, ECO:0000269|PubMed:27889207, ECO:0000269|PubMed:33693784, ECO:0000269|PubMed:7744004, ECO:0000269|PubMed:9171063}. |
 Retention analysis result of each fusion partner protein across 39 protein features of UniProt such as six molecule processing features, 13 region features, four site features, six amino acid modification features, two natural variation features, five experimental info features, and 3 secondary structure features. Here, because of limited space for viewing, we only show the protein feature retention information belong to the 13 regional features. All retention annotation result can be downloaded at * Minus value of BPloci means that the break pointn is located before the CDS. Retention analysis result of each fusion partner protein across 39 protein features of UniProt such as six molecule processing features, 13 region features, four site features, six amino acid modification features, two natural variation features, five experimental info features, and 3 secondary structure features. Here, because of limited space for viewing, we only show the protein feature retention information belong to the 13 regional features. All retention annotation result can be downloaded at * Minus value of BPloci means that the break pointn is located before the CDS. |
 - Retained domain in the 5'-partner of fusion protein (protein functional feature from UniProt). - Retained domain in the 5'-partner of fusion protein (protein functional feature from UniProt). |
| Partner | Hgeneene | Hbp | Tgeneene | Tbp | ENST | BPexon | TotalExon | Protein feature loci | BPloci | TotalLen | Feature | Note |
| Hgene | PAK1 | 77047130 | PAK2 | 196547409 | ENST00000530617 | 13 | 15 | 75_88 | 4711 | 546 | Domain | Note=CRIB;Ontology_term=ECO:0000255;evidence=ECO:0000255|PROSITE-ProRule:PRU00057 |
| Hgene | PAK1 | 77047130 | PAK2 | 196547409 | ENST00000530617 | 13 | 16 | 75_88 | 4711 | 554 | Domain | Note=CRIB;Ontology_term=ECO:0000255;evidence=ECO:0000255|PROSITE-ProRule:PRU00057 |
 - Retained domain in the 3'-partner of fusion protein (protein functional feature from UniProt). - Retained domain in the 3'-partner of fusion protein (protein functional feature from UniProt). |
| Partner | Hgeneene | Hbp | Tgeneene | Tbp | ENST | BPexon | TotalExon | Protein feature loci | BPloci | TotalLen | Feature | Note |
| Tgene | PAK1 | 77047130 | PAK2 | 196547409 | ENST00000530617 | 0 | 15 | 74_87 | 0 | 525 | Domain | Note=CRIB;Ontology_term=ECO:0000255;evidence=ECO:0000255|PROSITE-ProRule:PRU00057 |
| Tgene | PAK1 | 77047130 | PAK2 | 196547409 | ENST00000530617 | 0 | 15 | 249_499 | 0 | 525 | Domain | Note=Protein kinase;Ontology_term=ECO:0000255;evidence=ECO:0000255|PROSITE-ProRule:PRU00159 |
Top |
Kinase Fusion Protein Structures |
 CIF files of the predicted kinase fusion proteins CIF files of the predicted kinase fusion proteins * Here we show the 3D structure of the fusion proteins using Mol*. AlphaFold produces a per-residue confidence score (pLDDT) between 0 and 100. Model confidence is shown from the pLDDT values per residue. pLDDT corresponds to the model’s prediction of its score on the local Distance Difference Test. It is a measure of local accuracy (from AlphfaFold website). To color code individual residues, we transformed individual PDB files into CIF format. |
| Kinase Fusion protein CIF link (fusion AA seq ID in KinaseFusionDB) | Henst | Tenst | Hgene | Hchr | Hbp | Tgene | Tchr | Tbp | AA seq | Len(AA seq) |
| PDB file >>>189_PAK1_PAK2 | ENST00000530617 | ENST00000327134 | PAK1 | chr11 | 77047130 | PAK2 | chr3 | 196547409 | MLLVVTMSNNGLDIQDKPPAPPMRNTSTMIGAGSKDAGTLNHGSKPLPPNPEEKKKKDRFYRSILPGDKTNKKKEKERPEISLPSDFEHT IHVGFDAVTGEFTGMPEQWARLLQTSNITKSEQKKNPQAVLDVLEFYNSKKTSNSQKYMSFTDKSAEDYNSSNALNVKAVSETPAVPPVS EDEDDDDDDATPPPVIAPRPEHTKSVYTRSVIEPLPVTPTRDVATSPISPTENNTTPPDALTRNTEKQKKKPKMSDEEILEKLRSIVSVG DPKKKYTRFEKIGQGASGTVYTAMDVATGQEVAIKQMNLQQQPKKELIINEILVMRENKNPNIVNYLDSYLVGDELWVVMEYLAGGSLTD VVTETCMDEGQIAAVCRECLQALEFLHSNQVIHRDIKSDNILLGMDGSVKLTDFGFCAQITPEQSKRSTMVGTPYWMAPEVVTRKAYGPK | 485 |
| 3D view using mol* of 189_PAK1_PAK2 | ||||||||||
| PDB file >>>HKFP_275_PAK1_PAK2 | ENST00000530617 | ENST00000327134 | PAK1 | chr11 | 77047130 | PAK2 | chr3 | 196547409 | MLLVVTMSNNGLDIQDKPPAPPMRNTSTMIGAGSKDAGTLNHGSKPLPPNPEEKKKKDRFYRSILPGDKTNKKKEKERPEISLPSDFEHT IHVGFDAVTGEFTGMPEQWARLLQTSNITKSEQKKNPQAVLDVLEFYNSKKTSNSQKYMSFTDKSAEDYNSSNALNVKAVSETPAVPPVS EDEDDDDDDATPPPVIAPRPEHTKSVYTRSVIEPLPVTPTRDVATSPISPTENNTTPPDALTRNTEKQKKKPKMSDEEILEKLRSIVSVG DPKKKYTRFEKIGQGASGTVYTAMDVATGQEVAIKQMNLQQQPKKELIINEILVMRENKNPNIVNYLDSYLVGDELWVVMEYLAGGSLTD VVTETCMDEGQIAAVCRECLQALEFLHSNQVIHRDIKSDNILLGMDGSVKLTDFGFCAQITPEQSKRSTMVGTPYWMAPEVVTRKAYGPK | 485_PAK1_PAK2 |
| PDB file >>>359_PAK1_PAK2 | ENST00000530617 | ENST00000327134 | PAK1 | chr11 | 77047130 | PAK2 | chr3 | 196547409 | MLLVVTMSNNGLDIQDKPPAPPMRNTSTMIGAGSKDAGTLNHGSKPLPPNPEEKKKKDRFYRSILPGDKTNKKKEKERPEISLPSDFEHT IHVGFDAVTGEFTGMPEQWARLLQTSNITKSEQKKNPQAVLDVLEFYNSKKTSNSQKYMSFTDKSAEDYNSSNALNVKAVSETPAVPPVS EDEDDDDDDATPPPVIAPRPEHTKSVYTRSVIEPLPVTPTRDVATSPISPTENNTTPPDALTRNTEKQKKKPKMSDEEILEKLRSIVSVG DPKKKYTRFEKIGQGASGTVYTAMDVATGQEVAIKQMNLQQQPKKELIINEILVMRENKNPNIVNYLDSYLVGDELWVVMEYLAGGSLTD VVTETCMDEGQIAAVCRECLQALEFLHSNQVIHRDIKSDNILLGMDGSVKLTDFGFCAQITPEQSKRSTMVGTPYWMAPEVVTRKAYGPK | 485 |
| 3D view using mol* of 359_PAK1_PAK2 | ||||||||||
| PDB file >>>TKFP_614_PAK1_PAK2 | ENST00000530617 | ENST00000327134 | PAK1 | chr11 | 77047130 | PAK2 | chr3 | 196547409 | MLLVVTMSNNGLDIQDKPPAPPMRNTSTMIGAGSKDAGTLNHGSKPLPPNPEEKKKKDRFYRSILPGDKTNKKKEKERPEISLPSDFEHT IHVGFDAVTGEFTGMPEQWARLLQTSNITKSEQKKNPQAVLDVLEFYNSKKTSNSQKYMSFTDKSAEDYNSSNALNVKAVSETPAVPPVS EDEDDDDDDATPPPVIAPRPEHTKSVYTRSVIEPLPVTPTRDVATSPISPTENNTTPPDALTRNTEKQKKKPKMSDEEILEKLRSIVSVG DPKKKYTRFEKIGQGASGTVYTAMDVATGQEVAIKQMNLQQQPKKELIINEILVMRENKNPNIVNYLDSYLVGDELWVVMEYLAGGSLTD VVTETCMDEGQIAAVCRECLQALEFLHSNQVIHRDIKSDNILLGMDGSVKLTDFGFCAQITPEQSKRSTMVGTPYWMAPEVVTRKAYGPK | 485_PAK1_PAK2 |
Top |
Comparison of Fusion Protein Isoforms |
 Superimpose the 3D Structures Among All Fusion Protein Isoforms Superimpose the 3D Structures Among All Fusion Protein Isoforms * Download the pdb file and open it from the molstar online viewer. |
 Comparison of the Secondary Structures of Fusion Protein Isoforms Comparison of the Secondary Structures of Fusion Protein Isoforms |
Top |
Comparison of Fusion Protein Sequences/Structures with Known Sequences/Structures from PDB |
 Abstract of the Multiple Sequence Alignment of the Longest Fusion Protein Sequence and Known Sequence from PDB Search Using Fusion Gene Names Abstract of the Multiple Sequence Alignment of the Longest Fusion Protein Sequence and Known Sequence from PDB Search Using Fusion Gene Names  |
 Multiple Sequence Alignment of the Longest Fusion Protein Sequence and Known Sequence from PDB Search Using Fusion Gene Names Multiple Sequence Alignment of the Longest Fusion Protein Sequence and Known Sequence from PDB Search Using Fusion Gene Names |
 Superimpose the 3D Structures Between the Longest Fusion Protein and the Longest Known PDB Superimpose the 3D Structures Between the Longest Fusion Protein and the Longest Known PDB |
| 3D view using mol* of viewer/superimpose_pdbs/PAK1_PAK2_4EQC_superimposed.pdb.html | ||||||||||
| 3D view using mol* of viewer/superimpose_pdbs/PAK1_PAK2_5IME_superimposed.pdb.html |
Top |
pLDDT score distribution |
 pLDDT score distribution of the predicted fusion protein structures from AlphaFold2 pLDDT score distribution of the predicted fusion protein structures from AlphaFold2* AlphaFold produces a per-residue confidence score (pLDDT) between 0 and 100. * The blue color at the bottom marks the best active site residues. |
| 189_PAK1_PAK2.png |
 |
| 189_PAK1_PAK2.png |
 |
Top |
Potential Active Site Information |
 The potential binding sites of these fusion proteins were identified using SiteMap, a module of the Schrodinger suite. The potential binding sites of these fusion proteins were identified using SiteMap, a module of the Schrodinger suite. |
| Kinase Fusion AA seq ID in KinaseFusionDB | Site score | Size | Dscore | Volume | Exposure | Enclosure | Contact | Phobic | Philic | Balance | Don/Acc | Residues |
 Binding site residues of the found PDBs. Binding site residues of the found PDBs. |
| PDB accession | AA sequence | Residue position |
| 4EQC | ASP | 1 |
| 4EQC | PRO | 2 |
| 4EQC | PHE | 3 |
| 4EQC | THR | 4 |
| 4EQC | SER | 249 |
| 4EQC | ASP | 250 |
| 4EQC | GLU | 251 |
| 4EQC | GLU | 252 |
| 4EQC | ILE | 253 |
| 4EQC | LEU | 254 |
| 4EQC | GLU | 255 |
| 4EQC | LYS | 256 |
| 4EQC | LEU | 257 |
| 4EQC | ARG | 258 |
| 4EQC | SER | 259 |
| 4EQC | ILE | 260 |
| 4EQC | VAL | 261 |
| 4EQC | SER | 262 |
| 4EQC | VAL | 263 |
| 4EQC | GLY | 264 |
| 4EQC | ASP | 265 |
| 4EQC | PRO | 266 |
| 4EQC | LYS | 267 |
| 4EQC | LYS | 268 |
| 4EQC | LYS | 269 |
| 4EQC | TYR | 270 |
| 4EQC | THR | 271 |
| 4EQC | ARG | 272 |
| 4EQC | PHE | 273 |
| 4EQC | GLU | 274 |
| 4EQC | LYS | 275 |
| 4EQC | ILE | 276 |
| 4EQC | GLY | 277 |
| 4EQC | GLN | 278 |
| 4EQC | GLY | 279 |
| 4EQC | ALA | 280 |
| 4EQC | SER | 281 |
| 4EQC | GLY | 282 |
| 4EQC | THR | 283 |
| 4EQC | VAL | 284 |
| 4EQC | TYR | 285 |
| 4EQC | THR | 286 |
| 4EQC | ALA | 287 |
| 4EQC | MET | 288 |
| 4EQC | ASP | 289 |
| 4EQC | VAL | 290 |
| 4EQC | ALA | 291 |
| 4EQC | THR | 292 |
| 4EQC | GLY | 293 |
| 4EQC | GLN | 294 |
| 4EQC | GLU | 295 |
| 4EQC | VAL | 296 |
| 4EQC | ALA | 297 |
| 4EQC | ILE | 298 |
| 4EQC | ARG | 299 |
| 4EQC | GLN | 300 |
| 4EQC | MET | 301 |
| 4EQC | ASN | 302 |
| 4EQC | LEU | 303 |
| 4EQC | GLN | 304 |
| 4EQC | GLN | 305 |
| 4EQC | GLN | 306 |
| 4EQC | PRO | 307 |
| 4EQC | LYS | 308 |
| 4EQC | LYS | 309 |
| 4EQC | GLU | 310 |
| 4EQC | LEU | 311 |
| 4EQC | ILE | 312 |
| 4EQC | ILE | 313 |
| 4EQC | ASN | 314 |
| 4EQC | GLU | 315 |
| 4EQC | ILE | 316 |
| 4EQC | LEU | 317 |
| 4EQC | VAL | 318 |
| 4EQC | MET | 319 |
| 4EQC | ARG | 320 |
| 4EQC | GLU | 321 |
| 4EQC | ASN | 322 |
| 4EQC | LYS | 323 |
| 4EQC | ASN | 324 |
| 4EQC | PRO | 325 |
| 4EQC | ASN | 326 |
| 4EQC | ILE | 327 |
| 4EQC | VAL | 328 |
| 4EQC | ASN | 329 |
| 4EQC | TYR | 330 |
| 4EQC | LEU | 331 |
| 4EQC | ASP | 332 |
| 4EQC | SER | 333 |
| 4EQC | TYR | 334 |
| 4EQC | LEU | 335 |
| 4EQC | VAL | 336 |
| 4EQC | GLY | 337 |
| 4EQC | ASP | 338 |
| 4EQC | GLU | 339 |
| 4EQC | LEU | 340 |
| 4EQC | TRP | 341 |
| 4EQC | VAL | 342 |
| 4EQC | VAL | 343 |
| 4EQC | MET | 344 |
| 4EQC | GLU | 345 |
| 4EQC | TYR | 346 |
| 4EQC | LEU | 347 |
| 4EQC | ALA | 348 |
| 4EQC | GLY | 349 |
| 4EQC | GLY | 350 |
| 4EQC | SER | 351 |
| 4EQC | LEU | 352 |
| 4EQC | THR | 353 |
| 4EQC | ASP | 354 |
| 4EQC | VAL | 355 |
| 4EQC | VAL | 356 |
| 4EQC | THR | 357 |
| 4EQC | GLU | 358 |
| 4EQC | THR | 359 |
| 4EQC | CYS | 360 |
| 4EQC | MET | 361 |
| 4EQC | ASP | 362 |
| 4EQC | GLU | 363 |
| 4EQC | GLY | 364 |
| 4EQC | GLN | 365 |
| 4EQC | ILE | 366 |
| 4EQC | ALA | 367 |
| 4EQC | ALA | 368 |
| 4EQC | VAL | 369 |
| 4EQC | CYS | 370 |
| 4EQC | ARG | 371 |
| 4EQC | GLU | 372 |
| 4EQC | CYS | 373 |
| 4EQC | LEU | 374 |
| 4EQC | GLN | 375 |
| 4EQC | ALA | 376 |
| 4EQC | LEU | 377 |
| 4EQC | GLU | 378 |
| 4EQC | PHE | 379 |
| 4EQC | LEU | 380 |
| 4EQC | HIS | 381 |
| 4EQC | SER | 382 |
| 4EQC | ASN | 383 |
| 4EQC | GLN | 384 |
| 4EQC | VAL | 385 |
| 4EQC | ILE | 386 |
| 4EQC | HIS | 387 |
| 4EQC | ARG | 388 |
| 4EQC | ASP | 389 |
| 4EQC | ILE | 390 |
| 4EQC | LYS | 391 |
| 4EQC | SER | 392 |
| 4EQC | ASP | 393 |
| 4EQC | ASN | 394 |
| 4EQC | ILE | 395 |
| 4EQC | LEU | 396 |
| 4EQC | LEU | 397 |
| 4EQC | GLY | 398 |
| 4EQC | MET | 399 |
| 4EQC | ASP | 400 |
| 4EQC | GLY | 401 |
| 4EQC | SER | 402 |
| 4EQC | VAL | 403 |
| 4EQC | LYS | 404 |
| 4EQC | LEU | 405 |
| 4EQC | THR | 406 |
| 4EQC | ASP | 407 |
| 4EQC | PHE | 408 |
| 4EQC | GLY | 409 |
| 4EQC | PHE | 410 |
| 4EQC | CYS | 411 |
| 4EQC | ALA | 412 |
| 4EQC | GLN | 413 |
| 4EQC | ILE | 414 |
| 4EQC | THR | 415 |
| 4EQC | PRO | 416 |
| 4EQC | GLU | 417 |
| 4EQC | GLN | 418 |
| 4EQC | SER | 419 |
| 4EQC | LYS | 420 |
| 4EQC | ARG | 421 |
| 4EQC | SER | 422 |
| 4EQC | MET | 424 |
| 4EQC | VAL | 425 |
| 4EQC | GLY | 426 |
| 4EQC | THR | 427 |
| 4EQC | PRO | 428 |
| 4EQC | TYR | 429 |
| 4EQC | TRP | 430 |
| 4EQC | MET | 431 |
| 4EQC | ALA | 432 |
| 4EQC | PRO | 433 |
| 4EQC | GLU | 434 |
| 4EQC | VAL | 435 |
| 4EQC | VAL | 436 |
| 4EQC | THR | 437 |
| 4EQC | ARG | 438 |
| 4EQC | LYS | 439 |
| 4EQC | ALA | 440 |
| 4EQC | TYR | 441 |
| 4EQC | GLY | 442 |
| 4EQC | PRO | 443 |
| 4EQC | LYS | 444 |
| 4EQC | VAL | 445 |
| 4EQC | ASP | 446 |
| 4EQC | ILE | 447 |
| 4EQC | TRP | 448 |
| 4EQC | SER | 449 |
| 4EQC | LEU | 450 |
| 4EQC | GLY | 451 |
| 4EQC | ILE | 452 |
| 4EQC | MET | 453 |
| 4EQC | ALA | 454 |
| 4EQC | ILE | 455 |
| 4EQC | GLU | 456 |
| 4EQC | MET | 457 |
| 4EQC | ILE | 458 |
| 4EQC | GLU | 459 |
| 4EQC | GLY | 460 |
| 4EQC | GLU | 461 |
| 4EQC | PRO | 462 |
| 4EQC | PRO | 463 |
| 4EQC | TYR | 464 |
| 4EQC | LEU | 465 |
| 4EQC | ASN | 466 |
| 4EQC | GLU | 467 |
| 4EQC | ASN | 468 |
| 4EQC | PRO | 469 |
| 4EQC | LEU | 470 |
| 4EQC | ARG | 471 |
| 4EQC | ALA | 472 |
| 4EQC | LEU | 473 |
| 4EQC | TYR | 474 |
| 4EQC | LEU | 475 |
| 4EQC | ILE | 476 |
| 4EQC | ALA | 477 |
| 4EQC | THR | 478 |
| 4EQC | ASN | 479 |
| 4EQC | GLY | 480 |
| 4EQC | THR | 481 |
| 4EQC | PRO | 482 |
| 4EQC | GLU | 483 |
| 4EQC | LEU | 484 |
| 4EQC | GLN | 485 |
| 4EQC | ASN | 486 |
| 4EQC | PRO | 487 |
| 4EQC | GLU | 488 |
| 4EQC | LYS | 489 |
| 4EQC | LEU | 490 |
| 4EQC | SER | 491 |
| 4EQC | ALA | 492 |
| 4EQC | ILE | 493 |
| 4EQC | PHE | 494 |
| 4EQC | ARG | 495 |
| 4EQC | ASP | 496 |
| 4EQC | PHE | 497 |
| 4EQC | LEU | 498 |
| 4EQC | ASN | 499 |
| 4EQC | ARG | 500 |
| 4EQC | CYS | 501 |
| 4EQC | LEU | 502 |
| 4EQC | GLU | 503 |
| 4EQC | MET | 504 |
| 4EQC | ASP | 505 |
| 4EQC | VAL | 506 |
| 4EQC | GLU | 507 |
| 4EQC | LYS | 508 |
| 4EQC | ARG | 509 |
| 4EQC | GLY | 510 |
| 4EQC | SER | 511 |
| 4EQC | ALA | 512 |
| 4EQC | LYS | 513 |
| 4EQC | GLU | 514 |
| 4EQC | LEU | 515 |
| 4EQC | LEU | 516 |
| 4EQC | GLN | 517 |
| 4EQC | HIS | 518 |
| 4EQC | GLN | 519 |
| 4EQC | PHE | 520 |
| 4EQC | LEU | 521 |
| 4EQC | LYS | 522 |
| 4EQC | ILE | 523 |
| 4EQC | ALA | 524 |
| 4EQC | LYS | 525 |
| 4EQC | PRO | 526 |
| 4EQC | LEU | 527 |
| 4EQC | SER | 528 |
| 4EQC | SER | 529 |
| 4EQC | LEU | 530 |
| 4EQC | THR | 531 |
| 4EQC | PRO | 532 |
| 4EQC | LEU | 533 |
| 4EQC | ILE | 534 |
| 4EQC | ALA | 535 |
| 4EQC | ALA | 536 |
| 4EQC | ALA | 537 |
| 4EQC | LYS | 538 |
| 4EQC | GLU | 539 |
| 4EQC | ALA | 540 |
| 4EQC | THR | 541 |
| 5IME | GLU | 251 |
| 5IME | GLU | 252 |
| 5IME | ILE | 253 |
| 5IME | LEU | 254 |
| 5IME | GLU | 255 |
| 5IME | LYS | 256 |
| 5IME | LEU | 257 |
| 5IME | ARG | 258 |
| 5IME | SER | 259 |
| 5IME | ILE | 260 |
| 5IME | VAL | 261 |
| 5IME | SER | 262 |
| 5IME | VAL | 263 |
| 5IME | GLY | 264 |
| 5IME | ASP | 265 |
| 5IME | PRO | 266 |
| 5IME | LYS | 267 |
| 5IME | LYS | 275 |
| 5IME | ILE | 276 |
| 5IME | GLY | 277 |
| 5IME | GLN | 278 |
| 5IME | GLY | 279 |
| 5IME | ALA | 280 |
| 5IME | SER | 281 |
| 5IME | GLY | 282 |
| 5IME | THR | 283 |
| 5IME | VAL | 284 |
| 5IME | TYR | 285 |
| 5IME | THR | 286 |
| 5IME | ALA | 287 |
| 5IME | MET | 288 |
| 5IME | ASP | 289 |
| 5IME | GLN | 294 |
| 5IME | GLU | 295 |
| 5IME | VAL | 296 |
| 5IME | ALA | 297 |
| 5IME | ILE | 298 |
| 5IME | LYS | 299 |
| 5IME | GLN | 300 |
| 5IME | MET | 301 |
| 5IME | ASN | 302 |
| 5IME | LEU | 303 |
| 5IME | LYS | 309 |
| 5IME | GLU | 310 |
| 5IME | LEU | 311 |
| 5IME | ILE | 312 |
| 5IME | ILE | 313 |
| 5IME | ASN | 314 |
| 5IME | GLU | 315 |
| 5IME | ILE | 316 |
| 5IME | LEU | 317 |
| 5IME | VAL | 318 |
| 5IME | MET | 319 |
| 5IME | ARG | 320 |
| 5IME | GLU | 321 |
| 5IME | ASN | 322 |
| 5IME | LYS | 323 |
| 5IME | ASN | 324 |
| 5IME | PRO | 325 |
| 5IME | ASN | 326 |
| 5IME | ILE | 327 |
| 5IME | VAL | 328 |
| 5IME | ASN | 329 |
| 5IME | TYR | 330 |
| 5IME | LEU | 331 |
| 5IME | ASP | 332 |
| 5IME | SER | 333 |
| 5IME | TYR | 334 |
| 5IME | LEU | 335 |
| 5IME | VAL | 336 |
| 5IME | GLY | 337 |
| 5IME | ASP | 338 |
| 5IME | GLU | 339 |
| 5IME | LEU | 340 |
| 5IME | TRP | 341 |
| 5IME | VAL | 342 |
| 5IME | VAL | 343 |
| 5IME | MET | 344 |
| 5IME | GLU | 345 |
| 5IME | TYR | 346 |
| 5IME | LEU | 347 |
| 5IME | ALA | 348 |
| 5IME | GLY | 349 |
| 5IME | GLY | 350 |
| 5IME | SER | 351 |
| 5IME | LEU | 352 |
| 5IME | THR | 353 |
| 5IME | ASP | 354 |
| 5IME | VAL | 355 |
| 5IME | VAL | 356 |
| 5IME | THR | 357 |
| 5IME | GLU | 358 |
| 5IME | THR | 359 |
| 5IME | CYS | 360 |
| 5IME | MET | 361 |
| 5IME | ASP | 362 |
| 5IME | GLU | 363 |
| 5IME | GLY | 364 |
| 5IME | GLN | 365 |
| 5IME | ILE | 366 |
| 5IME | ALA | 367 |
| 5IME | ALA | 368 |
| 5IME | VAL | 369 |
| 5IME | CYS | 370 |
| 5IME | ARG | 371 |
| 5IME | GLU | 372 |
| 5IME | CYS | 373 |
| 5IME | LEU | 374 |
| 5IME | GLN | 375 |
| 5IME | ALA | 376 |
| 5IME | LEU | 377 |
| 5IME | GLU | 378 |
| 5IME | PHE | 379 |
| 5IME | LEU | 380 |
| 5IME | HIS | 381 |
| 5IME | SER | 382 |
| 5IME | ASN | 383 |
| 5IME | GLN | 384 |
| 5IME | VAL | 385 |
| 5IME | ILE | 386 |
| 5IME | HIS | 387 |
| 5IME | ARG | 388 |
| 5IME | ASN | 389 |
| 5IME | ILE | 390 |
| 5IME | LYS | 391 |
| 5IME | SER | 392 |
| 5IME | ASP | 393 |
| 5IME | ASN | 394 |
| 5IME | ILE | 395 |
| 5IME | LEU | 396 |
| 5IME | LEU | 397 |
| 5IME | GLY | 398 |
| 5IME | MET | 399 |
| 5IME | ASP | 400 |
| 5IME | GLY | 401 |
| 5IME | SER | 402 |
| 5IME | VAL | 403 |
| 5IME | LYS | 404 |
| 5IME | LEU | 405 |
| 5IME | THR | 406 |
| 5IME | ASP | 407 |
| 5IME | PHE | 408 |
| 5IME | GLY | 409 |
| 5IME | PHE | 410 |
| 5IME | CYS | 411 |
| 5IME | ALA | 412 |
| 5IME | GLN | 413 |
| 5IME | ILE | 414 |
| 5IME | THR | 415 |
| 5IME | PRO | 416 |
| 5IME | GLU | 417 |
| 5IME | GLN | 418 |
| 5IME | SER | 419 |
| 5IME | LYS | 420 |
| 5IME | ARG | 421 |
| 5IME | SER | 422 |
| 5IME | GLU | 423 |
| 5IME | MET | 424 |
| 5IME | VAL | 425 |
| 5IME | GLY | 426 |
| 5IME | THR | 427 |
| 5IME | PRO | 428 |
| 5IME | TYR | 429 |
| 5IME | TRP | 430 |
| 5IME | MET | 431 |
| 5IME | ALA | 432 |
| 5IME | PRO | 433 |
| 5IME | GLU | 434 |
| 5IME | VAL | 435 |
| 5IME | VAL | 436 |
| 5IME | THR | 437 |
| 5IME | ARG | 438 |
| 5IME | LYS | 439 |
| 5IME | ALA | 440 |
| 5IME | TYR | 441 |
| 5IME | GLY | 442 |
| 5IME | PRO | 443 |
| 5IME | LYS | 444 |
| 5IME | VAL | 445 |
| 5IME | ASP | 446 |
| 5IME | ILE | 447 |
| 5IME | TRP | 448 |
| 5IME | SER | 449 |
| 5IME | LEU | 450 |
| 5IME | GLY | 451 |
| 5IME | ILE | 452 |
| 5IME | MET | 453 |
| 5IME | ALA | 454 |
| 5IME | ILE | 455 |
| 5IME | GLU | 456 |
| 5IME | MET | 457 |
| 5IME | ILE | 458 |
| 5IME | GLU | 459 |
| 5IME | GLY | 460 |
| 5IME | GLU | 461 |
| 5IME | PRO | 462 |
| 5IME | PRO | 463 |
| 5IME | TYR | 464 |
| 5IME | LEU | 465 |
| 5IME | ASN | 466 |
| 5IME | GLU | 467 |
| 5IME | ASN | 468 |
| 5IME | PRO | 469 |
| 5IME | LEU | 470 |
| 5IME | ARG | 471 |
| 5IME | ALA | 472 |
| 5IME | LEU | 473 |
| 5IME | TYR | 474 |
| 5IME | LEU | 475 |
| 5IME | ILE | 476 |
| 5IME | ALA | 477 |
| 5IME | THR | 478 |
| 5IME | ASN | 479 |
| 5IME | GLY | 480 |
| 5IME | THR | 481 |
| 5IME | PRO | 482 |
| 5IME | GLU | 483 |
| 5IME | LEU | 484 |
| 5IME | GLN | 485 |
| 5IME | ASN | 486 |
| 5IME | PRO | 487 |
| 5IME | GLU | 488 |
| 5IME | LYS | 489 |
| 5IME | LEU | 490 |
| 5IME | SER | 491 |
| 5IME | ALA | 492 |
| 5IME | ILE | 493 |
| 5IME | PHE | 494 |
| 5IME | ARG | 495 |
| 5IME | ASP | 496 |
| 5IME | PHE | 497 |
| 5IME | LEU | 498 |
| 5IME | ASN | 499 |
| 5IME | ARG | 500 |
| 5IME | CYS | 501 |
| 5IME | LEU | 502 |
| 5IME | GLU | 503 |
| 5IME | MET | 504 |
| 5IME | ASP | 505 |
| 5IME | VAL | 506 |
| 5IME | GLU | 507 |
| 5IME | LYS | 508 |
| 5IME | ARG | 509 |
| 5IME | GLY | 510 |
| 5IME | SER | 511 |
| 5IME | ALA | 512 |
| 5IME | LYS | 513 |
| 5IME | GLU | 514 |
| 5IME | LEU | 515 |
| 5IME | LEU | 516 |
| 5IME | GLN | 517 |
| 5IME | HIS | 518 |
| 5IME | GLN | 519 |
| 5IME | PHE | 520 |
| 5IME | LEU | 521 |
| 5IME | LYS | 522 |
| 5IME | ILE | 523 |
| 5IME | ALA | 524 |
| 5IME | LYS | 525 |
| 5IME | PRO | 526 |
| 5IME | LEU | 527 |
| 5IME | SER | 528 |
| 5IME | SER | 529 |
| 5IME | LEU | 530 |
| 5IME | THR | 531 |
| 5IME | PRO | 532 |
| 5IME | LEU | 533 |
| 5IME | ILE | 534 |
| 5IME | ALA | 535 |
| 5IME | ALA | 536 |
| 5IME | ALA | 537 |
| 5IME | LYS | 538 |
| 5IME | GLU | 539 |
| 5IME | ALA | 540 |
| 5IME | THR | 541 |
| 5IME | LYS | 542 |
| 5IME | ASN | 543 |
| 5IME | ASN | 544 |
| 5IME | HIS | 545 |
| 5IME | GLY | 546 |
| 5IME | ASP | 250 |
| 5IME | GLU | 251 |
| 5IME | GLU | 252 |
| 5IME | ILE | 253 |
| 5IME | LEU | 254 |
| 5IME | GLU | 255 |
| 5IME | LYS | 256 |
| 5IME | LEU | 257 |
| 5IME | ARG | 258 |
| 5IME | SER | 259 |
| 5IME | ILE | 260 |
| 5IME | VAL | 261 |
| 5IME | SER | 262 |
| 5IME | VAL | 263 |
| 5IME | GLY | 264 |
| 5IME | ASP | 265 |
| 5IME | PRO | 266 |
| 5IME | LYS | 267 |
| 5IME | LYS | 268 |
| 5IME | LYS | 269 |
| 5IME | TYR | 270 |
| 5IME | THR | 271 |
| 5IME | ARG | 272 |
| 5IME | PHE | 273 |
| 5IME | GLU | 274 |
| 5IME | LYS | 275 |
| 5IME | ILE | 276 |
| 5IME | GLY | 277 |
| 5IME | GLN | 278 |
| 5IME | GLY | 279 |
| 5IME | ALA | 280 |
| 5IME | SER | 281 |
| 5IME | GLY | 282 |
| 5IME | THR | 283 |
| 5IME | VAL | 284 |
| 5IME | TYR | 285 |
| 5IME | THR | 286 |
| 5IME | ALA | 287 |
| 5IME | MET | 288 |
| 5IME | ASP | 289 |
| 5IME | VAL | 290 |
| 5IME | ALA | 291 |
| 5IME | THR | 292 |
| 5IME | GLY | 293 |
| 5IME | GLN | 294 |
| 5IME | GLU | 295 |
| 5IME | VAL | 296 |
| 5IME | ALA | 297 |
| 5IME | ILE | 298 |
| 5IME | LYS | 299 |
| 5IME | GLN | 300 |
| 5IME | MET | 301 |
| 5IME | ASN | 302 |
| 5IME | PRO | 307 |
| 5IME | LYS | 308 |
| 5IME | LYS | 309 |
| 5IME | GLU | 310 |
| 5IME | LEU | 311 |
| 5IME | ILE | 312 |
| 5IME | ILE | 313 |
| 5IME | ASN | 314 |
| 5IME | GLU | 315 |
| 5IME | ILE | 316 |
| 5IME | LEU | 317 |
| 5IME | VAL | 318 |
| 5IME | MET | 319 |
| 5IME | ARG | 320 |
| 5IME | GLU | 321 |
| 5IME | ASN | 322 |
| 5IME | LYS | 323 |
| 5IME | ASN | 324 |
| 5IME | PRO | 325 |
| 5IME | ASN | 326 |
| 5IME | ILE | 327 |
| 5IME | VAL | 328 |
| 5IME | ASN | 329 |
| 5IME | TYR | 330 |
| 5IME | LEU | 331 |
| 5IME | ASP | 332 |
| 5IME | SER | 333 |
| 5IME | TYR | 334 |
| 5IME | LEU | 335 |
| 5IME | VAL | 336 |
| 5IME | GLY | 337 |
| 5IME | ASP | 338 |
| 5IME | GLU | 339 |
| 5IME | LEU | 340 |
| 5IME | TRP | 341 |
| 5IME | VAL | 342 |
| 5IME | VAL | 343 |
| 5IME | MET | 344 |
| 5IME | GLU | 345 |
| 5IME | TYR | 346 |
| 5IME | LEU | 347 |
| 5IME | ALA | 348 |
| 5IME | GLY | 349 |
| 5IME | GLY | 350 |
| 5IME | SER | 351 |
| 5IME | LEU | 352 |
| 5IME | THR | 353 |
| 5IME | ASP | 354 |
| 5IME | VAL | 355 |
| 5IME | VAL | 356 |
| 5IME | THR | 357 |
| 5IME | GLU | 358 |
| 5IME | THR | 359 |
| 5IME | CYS | 360 |
| 5IME | MET | 361 |
| 5IME | ASP | 362 |
| 5IME | GLU | 363 |
| 5IME | GLY | 364 |
| 5IME | GLN | 365 |
| 5IME | ILE | 366 |
| 5IME | ALA | 367 |
| 5IME | ALA | 368 |
| 5IME | VAL | 369 |
| 5IME | CYS | 370 |
| 5IME | ARG | 371 |
| 5IME | GLU | 372 |
| 5IME | CYS | 373 |
| 5IME | LEU | 374 |
| 5IME | GLN | 375 |
| 5IME | ALA | 376 |
| 5IME | LEU | 377 |
| 5IME | GLU | 378 |
| 5IME | PHE | 379 |
| 5IME | LEU | 380 |
| 5IME | HIS | 381 |
| 5IME | SER | 382 |
| 5IME | ASN | 383 |
| 5IME | GLN | 384 |
| 5IME | VAL | 385 |
| 5IME | ILE | 386 |
| 5IME | HIS | 387 |
| 5IME | ARG | 388 |
| 5IME | ASN | 389 |
| 5IME | ILE | 390 |
| 5IME | LYS | 391 |
| 5IME | SER | 392 |
| 5IME | ASP | 393 |
| 5IME | ASN | 394 |
| 5IME | ILE | 395 |
| 5IME | LEU | 396 |
| 5IME | LEU | 397 |
| 5IME | GLY | 398 |
| 5IME | MET | 399 |
| 5IME | ASP | 400 |
| 5IME | GLY | 401 |
| 5IME | SER | 402 |
| 5IME | VAL | 403 |
| 5IME | LYS | 404 |
| 5IME | LEU | 405 |
| 5IME | THR | 406 |
| 5IME | ASP | 407 |
| 5IME | PHE | 408 |
| 5IME | GLU | 417 |
| 5IME | GLN | 418 |
| 5IME | SER | 419 |
| 5IME | LYS | 420 |
| 5IME | ARG | 421 |
| 5IME | SER | 422 |
| 5IME | GLU | 423 |
| 5IME | MET | 424 |
| 5IME | VAL | 425 |
| 5IME | GLY | 426 |
| 5IME | THR | 427 |
| 5IME | PRO | 428 |
| 5IME | TYR | 429 |
| 5IME | TRP | 430 |
| 5IME | MET | 431 |
| 5IME | ALA | 432 |
| 5IME | PRO | 433 |
| 5IME | GLU | 434 |
| 5IME | VAL | 435 |
| 5IME | VAL | 436 |
| 5IME | THR | 437 |
| 5IME | ARG | 438 |
| 5IME | LYS | 439 |
| 5IME | ALA | 440 |
| 5IME | TYR | 441 |
| 5IME | GLY | 442 |
| 5IME | PRO | 443 |
| 5IME | LYS | 444 |
| 5IME | VAL | 445 |
| 5IME | ASP | 446 |
| 5IME | ILE | 447 |
| 5IME | TRP | 448 |
| 5IME | SER | 449 |
| 5IME | LEU | 450 |
| 5IME | GLY | 451 |
| 5IME | ILE | 452 |
| 5IME | MET | 453 |
| 5IME | ALA | 454 |
| 5IME | ILE | 455 |
| 5IME | GLU | 456 |
| 5IME | MET | 457 |
| 5IME | ILE | 458 |
| 5IME | GLU | 459 |
| 5IME | GLY | 460 |
| 5IME | GLU | 461 |
| 5IME | PRO | 462 |
| 5IME | PRO | 463 |
| 5IME | TYR | 464 |
| 5IME | LEU | 465 |
| 5IME | ASN | 466 |
| 5IME | GLU | 467 |
| 5IME | ASN | 468 |
| 5IME | PRO | 469 |
| 5IME | LEU | 470 |
| 5IME | ARG | 471 |
| 5IME | ALA | 472 |
| 5IME | LEU | 473 |
| 5IME | TYR | 474 |
| 5IME | LEU | 475 |
| 5IME | ILE | 476 |
| 5IME | ALA | 477 |
| 5IME | THR | 478 |
| 5IME | ASN | 479 |
| 5IME | GLY | 480 |
| 5IME | THR | 481 |
| 5IME | PRO | 482 |
| 5IME | GLU | 483 |
| 5IME | LEU | 484 |
| 5IME | GLN | 485 |
| 5IME | ASN | 486 |
| 5IME | PRO | 487 |
| 5IME | GLU | 488 |
| 5IME | LYS | 489 |
| 5IME | LEU | 490 |
| 5IME | SER | 491 |
| 5IME | ALA | 492 |
| 5IME | ILE | 493 |
| 5IME | PHE | 494 |
| 5IME | ARG | 495 |
| 5IME | ASP | 496 |
| 5IME | PHE | 497 |
| 5IME | LEU | 498 |
| 5IME | ASN | 499 |
| 5IME | ARG | 500 |
| 5IME | CYS | 501 |
| 5IME | LEU | 502 |
| 5IME | GLU | 503 |
| 5IME | MET | 504 |
| 5IME | ASP | 505 |
| 5IME | VAL | 506 |
| 5IME | GLU | 507 |
| 5IME | LYS | 508 |
| 5IME | ARG | 509 |
| 5IME | GLY | 510 |
| 5IME | SER | 511 |
| 5IME | ALA | 512 |
| 5IME | LYS | 513 |
| 5IME | GLU | 514 |
| 5IME | LEU | 515 |
| 5IME | LEU | 516 |
| 5IME | GLN | 517 |
| 5IME | HIS | 518 |
| 5IME | GLN | 519 |
| 5IME | PHE | 520 |
| 5IME | LEU | 521 |
| 5IME | LYS | 522 |
| 5IME | ILE | 523 |
| 5IME | ALA | 524 |
| 5IME | LYS | 525 |
| 5IME | PRO | 526 |
| 5IME | LEU | 527 |
| 5IME | SER | 528 |
| 5IME | SER | 529 |
| 5IME | LEU | 530 |
| 5IME | THR | 531 |
| 5IME | PRO | 532 |
| 5IME | LEU | 533 |
| 5IME | ILE | 534 |
| 5IME | ALA | 535 |
| 5IME | ALA | 536 |
| 5IME | ALA | 537 |
| 5IME | LYS | 538 |
| 5IME | GLU | 539 |
| 5IME | ALA | 540 |
| 5IME | THR | 541 |
Top |
Ramachandran Plot of Kinase Fusion Protein Structure |
 Ramachandran plot of the torsional angles - phi (φ)and psi (ψ) - of the residues (amino acids) contained in this fusion protein peptide. Ramachandran plot of the torsional angles - phi (φ)and psi (ψ) - of the residues (amino acids) contained in this fusion protein peptide. |
| 189_PAK1_PAK2_ramachandran.png |
 |
Top |
Virtual Screening Results |
 Distribution of the average docking score across all approved kinase inhibitors. Distribution of the average docking score across all approved kinase inhibitors.Distribution of the number of occurrence across all approved kinase inhibitors. |
| 5'-kinase fusion protein case |
 |
| 3'-kinase fusion protein case |
Top |
 Drug information from DrugBank of the top 20 interacting small molecules. Drug information from DrugBank of the top 20 interacting small molecules.* The detailed information of individual kinase inhibitors are available in the download page. |
| Fusion gene name info | Drug | Docking score | Glide g score | Glide energy |
| 189_PAK1_PAK2-DOCK_HTVS_1-001 | Pralsetinib | -8.47723 | -8.56873 | -43.7312 |
| 189_PAK1_PAK2-DOCK_HTVS_1-001 | Acalabrutinib | -7.51242 | -7.52652 | -38.8977 |
| 189_PAK1_PAK2-DOCK_HTVS_1-001 | Acalabrutinib | -7.51242 | -7.52652 | -38.8977 |
| 189_PAK1_PAK2-DOCK_HTVS_1-001 | Midostaurin | -7.3859699999999995 | -7.3859699999999995 | -43.0655 |
| 189_PAK1_PAK2-DOCK_HTVS_1-001 | Neratinib | -6.982480000000001 | -7.16838 | -60.2967 |
| 189_PAK1_PAK2-DOCK_HTVS_1-001 | Neratinib | -6.94039 | -7.1226899999999995 | -59.2826 |
| 189_PAK1_PAK2-DOCK_HTVS_1-001 | Neratinib | -6.94039 | -7.1226899999999995 | -59.2826 |
| 189_PAK1_PAK2-DOCK_HTVS_1-001 | Midostaurin | -6.901860000000001 | -6.901860000000001 | -39.0018 |
| 189_PAK1_PAK2-DOCK_HTVS_1-001 | Larotrectinib | -6.82308 | -6.82308 | -33.4717 |
| 189_PAK1_PAK2-DOCK_HTVS_1-001 | Lapatinib | -6.742999999999999 | -6.8318 | -57.4458 |
| 189_PAK1_PAK2-DOCK_HTVS_1-001 | Lapatinib | -6.71192 | -6.80072 | -57.7045 |
| 189_PAK1_PAK2-DOCK_HTVS_1-001 | Larotrectinib | -6.6882399999999995 | -6.6882399999999995 | -37.8026 |
| 189_PAK1_PAK2-DOCK_HTVS_1-001 | Encorafenib | -6.600060000000001 | -6.9885600000000005 | -46.4323 |
| 189_PAK1_PAK2-DOCK_HTVS_1-001 | Lenvatinib | -6.506530000000001 | -6.506530000000001 | -41.4377 |
| 189_PAK1_PAK2-DOCK_HTVS_1-001 | Zanubrutinib | -6.505719999999999 | -6.505719999999999 | -46.6558 |
| 189_PAK1_PAK2-DOCK_HTVS_1-001 | Midostaurin | -6.42277 | -6.42277 | -30.2677 |
| 189_PAK1_PAK2-DOCK_HTVS_1-001 | Binimetinib | -6.3872 | -6.3959 | -48.0055 |
| 189_PAK1_PAK2-DOCK_HTVS_1-001 | Binimetinib | -6.3872 | -6.3959 | -48.0055 |
| 189_PAK1_PAK2-DOCK_HTVS_1-001 | Selpercatinib | -6.37918 | -6.409680000000001 | -41.7146 |
| 189_PAK1_PAK2-DOCK_HTVS_1-001 | Belumosudil | -6.34066 | -6.34836 | -45.8752 |
Top |
Kinase-Substrate Information of PAK1_PAK2 |
 Phosphorylation target of the kinase Phosphorylation target of the kinase(phosphosite, 03-17-2024) |
| Kinase | Kinase UniProt Acc | Kinase species | Substrate | Substrate UniProt Acc | Substrate phosphorylated residues | Substrate phosphorylated sites (+/-7AA) | Domain |
| PAK1 | Q13153 | human | FXR1 | P51114 | S420 | sERkDELsDWsLAGE | FXMRP1_C_core |
| PAK1 | Q13153 | human | ARPC1B | O15143 | T21 | HAWNkDRtQIAICPN | |
| PAK1 | Q13153 | human | GNAZ | P19086 | S16 | EKEAARRsRRIDRHL | G-alpha |
| PAK1 | Q13153 | human | ARHGEF2 | Q92974 | S886 | PVDPRRRsLPAGDAL | |
| PAK1 | Q13153 | human | MAP3K1 | Q13233 | S67 | RQLRKVRsVELDQLP | |
| PAK1 | Q13153 | human | TBCB | Q99426 | S128 | VRsFLKRsKLGRyNE | |
| PAK1 | Q13153 | human | CRK | P46108 | S41 | VFLVRDsstsPGDyV | SH2 |
| PAK1 | Q13153 | human | CTNNB1 | P35222 | S675 | QDykKRLsVELtsSL | |
| PAK1 | Q13153 | human | GDI2 | P50395 | T248 | LSAIyGGtYMLNkPI | GDI |
| PAK1 | Q13153 | human | MICAL1 | Q8TDZ2 | S817 | sPERQRLssLNLtPD | |
| PAK1 | Q13153 | human | PXN | P49023 | S258 | tQQQtRIsAssAtRE | |
| PAK1 | Q13153 | human | GDI2 | P50395 | S382 | PIEQkFVsIsDLLVP | GDI |
| PAK1 | Q13153 | human | TBCB | Q99426 | S65 | GVDDKFYskLDQEDA | Ubiquitin_2 |
| PAK1 | Q13153 | human | PXN | P49023-2 | S272 | ELDELMAsLSDFKFM | |
| PAK1 | Q13153 | human | PLK1 | P53350 | S49 | EVLVDPRsRRRYVRG | |
| PAK1 | Q13153 | human | H3C1 | P68431 | S10 | tkQtArkstGGkAPr | Histone |
| PAK1 | Q13153 | human | PAK1 | Q13153 | S204 | tKsVyTRsVIEPLPV | |
| PAK1 | Q13153 | human | SNAI1 | O95863 | S246 | QACARTFsRMsLLHK | |
| PAK1 | Q13153 | human | FLNA | P21333 | S2292 | FEDRkDGsCGVAYVV | Filamin |
| PAK1 | Q13153 | human | ARHGDIA | P52565 | S174 | kGMLARGsYsIkSRF | Rho_GDI |
| PAK1 | Q13153 | human | PCBP1 | Q15365 | T60 | NCPERIItLTGPTNA | KH_1 |
| PAK1 | Q13153 | human | CTTN | Q14247 | S405 | KtQtPPVsPAPQPtE | |
| PAK1 | Q13153 | human | DYNLL1 | P63167 | S88 | VAILLFKsG______ | Dynein_light |
| PAK1 | Q13153 | human | ARL4A | P40617 | S143 | QDLRNSLsLSEIEKL | Arf |
| PAK1 | Q13153 | human | NLRP3 | Q96P20 | S198 | GKTKTCEsPVSPIKM | FISNA |
| PAK1 | Q13153 | human | ARL4D | P49703 | S144 | QDQPGALsAAEVEkR | Arf |
| PAK1 | Q13153 | human | SPEN | Q96T58 | T3568 | EGVARRMtVETDYCL | SPOC |
| PAK1 | Q13153 | human | ARHGEF2 | Q92974-2 | S885 | PVDPRRRsLPAGDAL | |
| PAK1 | Q13153 | human | AURKA | O14965 | S342 | QETYKRIsRVEFTFP | Pkinase |
| PAK1 | Q13153 | human | STMN1 | P16949 | S38 | sVPEFPLsPPkKkDL | Stathmin |
| PAK1 | Q13153 | human | GIT1 | Q9Y2X7 | S700 | PALEPVRsSLRLLNA | GIT1_C |
| PAK1 | Q13153 | human | PCBP1 | Q15365 | T127 | IKEIREStGAQVQVA | KH_1 |
| PAK1 | Q13153 | human | NF2 | P35240 | S518 | DTDMKRLsMEIEKEK | ERM_C |
| PAK1 | Q13153 | human | PA2G4 | Q9UQ80 | T261 | QyGLkMktSRAFFsE | Peptidase_M24 |
| PAK1 | Q13153 | human | RUNX3 | Q13761 | T209 | ERLRMRVtPstPsPr | |
| PAK1 | Q13153 | human | ARHGDIA | P52565 | S101 | LEsFKkQsFVLkEGV | Rho_GDI |
| PAK1 | Q13153 | human | STMN1 | P16949 | S16 | kELEKrAsGQAFELI | Stathmin |
| PAK1 | Q13153 | human | MAP2K1 | Q02750 | S298 | RtPGRPLssyGMDSR | Pkinase |
| PAK1 | Q13153 | human | BAD | Q92934 | S99 | PFrGrsRsAPPNLWA | Bcl-2_BAD |
| PAK1 | Q13153 | human | KIF2C | Q99661 | S111 | kEsLRsRstRMstVS | |
| PAK1 | Q13153 | human | AURKA | O14965 | T288 | APSsRRttLCGtLDy | Pkinase |
| PAK1 | Q13153 | human | PGM1 | P36871 | T467 | SANDKVYtVEkADNF | |
| PAK1 | Q13153 | human | PAK1 | Q13153 | S199 | PRPEHtKsVyTRsVI | |
| PAK1 | Q13153 | human | RAF1 | P04049 | S339 | PRGQRDssyyWEIEA | |
| PAK1 | Q13153 | human | ILK | Q13418 | S246 | CPRLRIFsHPNVLPV | PK_Tyr_Ser-Thr |
| PAK1 | Q13153 | human | BAD | Q92934 | S75 | EIRsRHssyPAGtED | Bcl-2_BAD |
| PAK1 | Q13153 | human | KIF2C | Q99661 | S192 | VNsVRRKsCLVkEVE | |
| PAK1 | Q13153 | human | PGAM1 | P18669 | S23 | WNLENrFsGWyDADL | His_Phos_1 |
| PAK1 | Q13153 | human | ARHGDIA | P52565 | S176 | MLARGsYsIkSRFTD | Rho_GDI |
| PAK1 | Q13153 | human | ELF3 | P78545 | S207 | GTGASRSsHSSDsGG | |
| PAK1 | Q13153 | human | MORC2 | Q9Y6X9 | S739 | AtPsRKRsVAVsDEE | |
| PAK1 | Q13153 | human | CTTN | Q14247 | S113 | skLskHCsQVDsVrG | HS1_rep |
| PAK1 | Q13153 | human | NLRP3 | Q96P20 | T659 | KIEINLStRMDHMVS | |
| PAK1 | Q13153 | human | BAD | Q92934 | S118 | GRELRRMsDEFVDsF | Bcl-2_BAD |
| PAK1 | Q13153 | human | GIT1 | Q9Y2X7 | S508 | RRDRQAFsMyEPGsA | |
| PAK1 | Q13153 | human | ITGB3BP | Q13352 | S28 | SkITRKKsVITysPT | CENP-R |
| PAK1 | Q13153 | human | BAD | Q92934 | S74 | VEIRsRHssyPAGtE | Bcl-2_BAD |
| PAK1 | Q13153 | human | NLRP3 | Q96P20 | S163 | ARLGEsVsLNKRYTR | FISNA |
| PAK1 | Q13153 | human | MYL9 | P24844 | S20 | KRPQRAtsNVFAMFD | |
| PAK1 | Q13153 | human | FLNA | P21333 | S2152 | tRRRRAPsVANVGsH | Filamin |
| PAK1 | Q13153 | human | SNAI2 | O43623 | S251 | KNCsKTFsRMsLLHK | zf-C2H2 |
| PAK1 | Q13153 | human | FLNA | P21333 | S2370 | AIDAkVHsPsGALEE | Filamin |
| PAK1 | Q13153 | human | BRAF | P15056 | S446 | KtLGRRDssDDWEIP | |
| PAK1 | Q13153 | human | PGAM1 | P18669 | S118 | QVkIWRRsyDVPPPP | His_Phos_1 |
| PAK1 | Q13153 | human | CTNNB1 | P35222 | S552 | QDtQRRtsMGGtQQQ | |
| PAK1 | Q13153 | human | SPEN | Q96T58 | S3486 | QPAPKQDssPHLTSQ | |
| PAK1 | Q13153 | human | CTTN | Q14247 | S418 | tEErLPssPVyEDAA | |
| PAK1 | Q13153 | human | LIMK1 | P53667 | T508 | PDRKKRYtVVGNPYW | PK_Tyr_Ser-Thr |
| PAK1 | Q13153 | human | PAK1 | Q13153 | S144 | sNsQKyMsFtDksAE | |
| PAK1 | Q13153 | human | RAF1 | P04049 | S338 | RPRGQRDssyyWEIE | |
| PAK1 | Q13153 | human | PREX2 | Q70Z35 | S1107 | DTISNRDsYSDCNSN | |
| PAK1 | Q13153 | human | ILK | Q13418 | T173 | DTFWkGttRTRPRNG | |
| PAK1 | Q13153 | human | CTBP1 | Q13363 | S158 | REGTRVQsVEQIREV | 2-Hacid_dh_C; 2-Hacid_dh |
| PAK1 | Q13153 | human | PAK1 | Q13153 | T423 | PEQSkRstMVGtPYW | Pkinase |
| PAK1 | Q13153 | human | MICAL1 | Q8TDZ2 | S960 | ELALRRQssSPEQQK | bMERB_dom |
| PAK1 | Q13153 | human | HACE1 | Q8IYU2 | S385 | LMKNKRDsTEITsIL | |
| PAK1 | Q13153 | human | ESR1 | P03372 | S305 | IkRSkkNsLALSLtA | |
| PAK1 | Q13153 | human | MYLK | Q15746 | S1772 | SGLsGRKsstGsPts | |
| PAK1 | Q13153 | human | BAD | Q92934 | S134 | KGLPRPKsAGtAtQM | Bcl-2_BAD |
| PAK1 | Q13153 | human | VIM | P08670 | S56 | srsLyAssPGGVyAt | Filament_head |
| PAK1 | Q13153 | human | ATG5 | Q9H1Y0 | T101 | SALPWNItVHFKSFP | APG5 |
| PAK2 | Q13177 | human | MYC | P01106 | S388 | RRNELkRsFFALRDQ | HLH |
| PAK2 | Q13177 | human | ARHGAP15 | Q53QZ3 | S292 | kVCERENsTVPWFVk | |
| PAK2 | Q13177 | human | H4C1 | P62805 | S47 | RGGVKrIsGLIyEEt | CENP-T_C |
| PAK2 | Q13177 | human | JUN | P05412 | T2 | ______MtAKMETtF | |
| PAK2 | Q13177 | human | CALD1 | Q05682 | S744 | GLKVGVSsRINEWLT | Caldesmon |
| PAK2 | Q13177 | human | JUN | P05412 | T286 | RLEEKVKtLKAQNSE | bZIP_1 |
| PAK2 | Q13177 | human | MYC | P01106 | T373 | EENVkRRtHNVLERQ | HLH |
| PAK2 | Q13177 | human | MICAL1 | Q8TDZ2 | S817 | sPERQRLssLNLtPD | |
| PAK2 | Q13177 | human | SORT1 | Q99523 | S793 | RFLVHRYsVLQQHAE | |
| PAK2 | Q13177 | human | CASP7 | P55210 | S239 | WRsPGRGsWFVQALC | Peptidase_C14 |
| PAK2 | Q13177 | human | ABL1 | P00519 | S619 | PtPPKRsssFREMDG | |
| PAK2 | Q13177 | human | PAK2 | Q13177 | S192 | PRPDHTksIytRsVI | |
| PAK2 | Q13177 | human | PAK2 | Q13177 | S141 | tVkQKyLsFtPPEkd | |
| PAK2 | Q13177 | human | SMAD2 | Q15796 | S467 | sVRCssMs_______ | |
| PAK2 | Q13177 | human | NF2 | P35240 | S518 | DTDMKRLsMEIEKEK | ERM_C |
| PAK2 | Q13177 | human | SMAD2 | Q15796 | S417 | RMCTIRMsFVkGWGA | MH2 |
| PAK2 | Q13177 | human | EIF4G1 | Q04637 | S895 | RDIARRRsLGNIkFI | MIF4G |
| PAK2 | Q13177 | human | ARHGAP15 | Q53QZ3 | S4 | ____MQKsTNsDTSV | |
| PAK2 | Q13177 | human | ARHGAP15 | Q53QZ3 | S43 | DRLsQsksMILTDVG | |
| PAK2 | Q13177 | human | PAK2 | Q13177 | T402 | PEQSkRstMVGtPYW | Pkinase |
| PAK2 | Q13177 | human | JUN | P05412 | T8 | MtAKMETtFYDDALN | Jun |
| PAK2 | Q13177 | human | SMAD2 | Q15796 | S465 | sPsVRCssMs_____ | |
| PAK2 | Q13177 | human | MYC | P01106 | T415 | VVILKkAtAYILsVQ | HLH |
| PAK2 | Q13177 | human | PXN | P49023 | S272 | ELDELMAsLsDFkIQ | |
| PAK2 | Q13177 | human | CASP7 | P55210 | S30 | DAKPDRssFVPsLFs | |
| PAK2 | Q13177 | human | CALD1 | Q05682 | S714 | EGVRNIksMWEkGNV | Caldesmon |
| PAK2 | Q13177 | human | ARHGAP15 | Q53QZ3 | S41 | HHDRLsQsksMILTD | |
| PAK2 | Q13177 | human | MAPK6 | Q16659 | S189 | ySHKGHLsEGLVTkW | Pkinase |
| PAK2 | Q13177 | human | PXN | P49023 | S274 | DELMAsLsDFkIQGL | |
| PAK2 | Q13177 | human | PAK2 | Q13177 | S197 | TksIytRsVIDPVPA | |
| PAK2 | Q13177 | human | CASP7 | P55210 | T173 | FRGDRCktLLEkPKL | Peptidase_C14 |
| PAK2 | Q13177 | human | CTNNB1 | P35222 | S552 | QDtQRRtsMGGtQQQ | |
| PAK2 | Q13177 | human | SMAD2 | Q15796 | S464 | GsPsVRCssMs____ | |
| PAK2 | Q13177 | human | JUN | P05412 | T89 | QSSNGHItTtPtPtQ | Jun |
| PAK2 | Q13177 | human | PREX2 | Q70Z35 | S1107 | DTISNRDsYSDCNSN | |
| PAK2 | Q13177 | human | RAF1 | P04049 | S338 | RPRGQRDssyyWEIE | |
| PAK2 | Q13177 | human | MICAL1 | Q8TDZ2 | S960 | ELALRRQssSPEQQK | bMERB_dom |
| PAK2 | Q13177 | human | HACE1 | Q8IYU2 | S385 | LMKNKRDsTEITsIL | |
| PAK2 | Q13177 | human | SLC25A5 | P05141 | T107 | LGGVDkRtQFWLyFA | |
| PAK2 | Q13177 | human | JUN | P05412 | T93 | GHItTtPtPtQFLCP | Jun |
| PAK2 | Q13177 | human | ABL1 | P00519 | S618 | APtPPKRsssFREMD |
 Biological Network Integration of This Kinase and Substrates Biological Network Integration of This Kinase and Substrates (GeneMANIA website) |
 Enriched GO biological processes of the phosphorylation target genes of the kinase Enriched GO biological processes of the phosphorylation target genes of the kinase |
| Kinase | GOID | GO term | P.adjust |
| PAK1 | ID | Description | 0.00e+00 |
| PAK1 | GO:0051017 | actin filament bundle assembly | 7.15e-06 |
| PAK1 | GO:0061572 | actin filament bundle organization | 7.15e-06 |
| PAK1 | GO:0032233 | positive regulation of actin filament bundle assembly | 7.15e-06 |
| PAK1 | GO:0032231 | regulation of actin filament bundle assembly | 7.15e-06 |
| PAK1 | GO:1902905 | positive regulation of supramolecular fiber organization | 8.29e-06 |
| PAK1 | GO:0051495 | positive regulation of cytoskeleton organization | 9.71e-06 |
| PAK1 | GO:0007015 | actin filament organization | 9.71e-06 |
| PAK1 | GO:1902903 | regulation of supramolecular fiber organization | 1.82e-05 |
| PAK1 | GO:0051492 | regulation of stress fiber assembly | 4.26e-05 |
| PAK1 | GO:0051496 | positive regulation of stress fiber assembly | 4.66e-05 |
| PAK1 | GO:0032956 | regulation of actin cytoskeleton organization | 4.66e-05 |
| PAK1 | GO:0110020 | regulation of actomyosin structure organization | 6.18e-05 |
| PAK1 | GO:0030038 | contractile actin filament bundle assembly | 6.98e-05 |
| PAK1 | GO:0043149 | stress fiber assembly | 6.98e-05 |
| PAK1 | GO:0007264 | small GTPase mediated signal transduction | 7.90e-05 |
| PAK1 | GO:0110053 | regulation of actin filament organization | 7.90e-05 |
| PAK1 | GO:0032970 | regulation of actin filament-based process | 8.07e-05 |
| PAK1 | GO:0031032 | actomyosin structure organization | 1.50e-04 |
| PAK1 | GO:0010720 | positive regulation of cell development | 2.67e-04 |
| PAK1 | GO:0007265 | Ras protein signal transduction | 3.33e-04 |
| PAK1 | GO:0031589 | cell-substrate adhesion | 4.35e-04 |
| PAK1 | GO:1900026 | positive regulation of substrate adhesion-dependent cell spreading | 4.38e-04 |
| PAK1 | GO:0022411 | cellular component disassembly | 5.51e-04 |
| PAK1 | GO:1903034 | regulation of response to wounding | 5.70e-04 |
| PAK1 | GO:0034446 | substrate adhesion-dependent cell spreading | 5.74e-04 |
| PAK1 | GO:0048538 | thymus development | 6.92e-04 |
| PAK1 | GO:0046578 | regulation of Ras protein signal transduction | 6.98e-04 |
| PAK1 | GO:0071470 | cellular response to osmotic stress | 8.16e-04 |
| PAK1 | GO:0051056 | regulation of small GTPase mediated signal transduction | 9.24e-04 |
| PAK1 | GO:1900024 | regulation of substrate adhesion-dependent cell spreading | 1.44e-03 |
| PAK1 | GO:0048732 | gland development | 1.44e-03 |
| PAK1 | GO:0031109 | microtubule polymerization or depolymerization | 1.86e-03 |
| PAK1 | GO:0007266 | Rho protein signal transduction | 2.00e-03 |
| PAK1 | GO:0061311 | cell surface receptor signaling pathway involved in heart development | 2.67e-03 |
| PAK1 | GO:2001234 | negative regulation of apoptotic signaling pathway | 2.67e-03 |
| PAK1 | GO:0044342 | type B pancreatic cell proliferation | 3.17e-03 |
| PAK1 | GO:0032886 | regulation of microtubule-based process | 3.47e-03 |
| PAK1 | GO:0006970 | response to osmotic stress | 3.47e-03 |
| PAK1 | GO:0039694 | viral RNA genome replication | 3.47e-03 |
| PAK1 | GO:0048679 | regulation of axon regeneration | 3.47e-03 |
| PAK1 | GO:0070507 | regulation of microtubule cytoskeleton organization | 3.47e-03 |
| PAK1 | GO:0035023 | regulation of Rho protein signal transduction | 3.47e-03 |
| PAK1 | GO:0030878 | thyroid gland development | 3.60e-03 |
| PAK1 | GO:0050767 | regulation of neurogenesis | 3.60e-03 |
| PAK1 | GO:0061351 | neural precursor cell proliferation | 3.66e-03 |
| PAK1 | GO:0070570 | regulation of neuron projection regeneration | 3.72e-03 |
| PAK1 | GO:2001233 | regulation of apoptotic signaling pathway | 3.83e-03 |
| PAK1 | GO:0031110 | regulation of microtubule polymerization or depolymerization | 5.14e-03 |
| PAK1 | GO:0042060 | wound healing | 5.36e-03 |
Top |
Related Drugs to PAK1_PAK2 |
 Drugs used for this fusion-positive patient. Drugs used for this fusion-positive patient. (Manual curation of PubMed, 04-30-2022 + MyCancerGenome) |
| Hgene | Tgene | Drug | Source | PMID |
 Distribution of the number of studies mentioning PAK1-PAK2 and kinase inhibitors the PubMed Abstract (04-01-2024) Distribution of the number of studies mentioning PAK1-PAK2 and kinase inhibitors the PubMed Abstract (04-01-2024) |
| Fusion gene - drug pair 1 | Fusion gene - drug pair 2 | PMID | Publication date | DOI | Study title |
Top |
Related Diseases to PAK1_PAK2 |
 Diseases that have this fusion gene. Diseases that have this fusion gene. (Manual curation of PubMed, 04-30-2022 + MyCancerGenome) |
| Hgene | Tgene | Disease | Source | PMID |
 Related diseases from the literature mentioned this fusion gene and drug. Related diseases from the literature mentioned this fusion gene and drug. (PubMed, 04-01-2024) |
| MeSH ID | MeSH term |
 Diseases associated with fusion partners. Diseases associated with fusion partners. (DisGeNet 4.0) |
| Partner | Gene | Disease ID | Disease name | # pubmeds | Source |
Top |
Clinical Trials of the Found Drugs/Small Molecules |
 Statistics of the Clinical Trials of the Found Kinase Inibitors from clinicaltrials.gov (06-17-2024) Statistics of the Clinical Trials of the Found Kinase Inibitors from clinicaltrials.gov (06-17-2024) |
 Clinical Trials from clinicaltrials.gov (06-17-2024) Clinical Trials from clinicaltrials.gov (06-17-2024) |
| Fusion Gene | Kinase Inhibitor | NCT ID | Study Status | Phases | Disease | # Enrolment | Date |

