| UTHEALTH HOME ABOUT SBMI A-Z WEBMAIL INSIDE THE UNIVERSITY |

|
|||||||
|
Kinase Fusion Gene:PRKAA1_NIPBL |
Kinase Fusion Protein Summary |
 Kinase Fusion gene summary Kinase Fusion gene summary |
| Kinase Fusion partner gene information | Kinase Fusion gene name: PRKAA1_NIPBL | KinaseFusionDB ID: KFG4856 | FusionGDB2.0 ID: KFG4856 | Hgene | Tgene | Gene symbol | PRKAA1 | NIPBL | Gene ID | 5562 | 25836 | |
| Gene name | protein kinase AMP-activated catalytic subunit alpha 1 | NIPBL cohesin loading factor | ||||||||||
| Synonyms | AMPK|AMPK alpha 1|AMPKa1 | CDLS|CDLS1|IDN3|IDN3-B|Scc2 | ||||||||||
| Cytomap | 5p13.1 | 5p13.2 | ||||||||||
| Type of gene | protein-coding | protein-coding | ||||||||||
| Description | 5'-AMP-activated protein kinase catalytic subunit alpha-15'-AMP-activated protein kinase, catalytic alpha-1 chainACACA kinaseAMP -activate kinase alpha 1 subunitAMP-activated protein kinase, catalytic, alpha-1AMPK subunit alpha-1AMPK, alpha, 1HMGCR | nipped-B-like proteinNipped-B homologSCC2 homologdelanginsister chromatid cohesion 2 homolog | ||||||||||
| Modification date | 20240408 | 20240407 | ||||||||||
| UniProtAcc | Q13131 | Q6KC79 | ||||||||||
| Ensembl transtripts involved in fusion gene | ENST ids | ENST00000397128, ENST00000354209, ENST00000296800, | ENST00000282516, ENST00000448238, ENST00000504430, | |||||||||
| Context (manual curation of fusion genes in KinaseFusionDB) | PubMed: PRKAA1 [Title/Abstract] AND NIPBL [Title/Abstract] AND fusion [Title/Abstract] | |||||||||||
| Most frequent breakpoint (based on all fusion genes of FusionGDB 2.0) | ||||||||||||
 Gene ontology of each fusion partner gene with evidence of Inferred from Direct Assay (IDA) from Entrez Gene ontology of each fusion partner gene with evidence of Inferred from Direct Assay (IDA) from Entrez |
| Partner | Gene | GO ID | GO term | PubMed ID |
| Hgene | PRKAA1 | GO:0006468 | protein phosphorylation | 17028174 |
| Hgene | PRKAA1 | GO:0010508 | positive regulation of autophagy | 22012985|25891078 |
| Hgene | PRKAA1 | GO:0010628 | positive regulation of gene expression | 17028174 |
| Hgene | PRKAA1 | GO:0031669 | cellular response to nutrient levels | 25412657|30478170 |
| Hgene | PRKAA1 | GO:0042149 | cellular response to glucose starvation | 14651849|18439900|34077757|36732624 |
| Hgene | PRKAA1 | GO:1903944 | negative regulation of hepatocyte apoptotic process | 32029622 |
| Hgene | PRKAA1 | GO:1904262 | negative regulation of TORC1 signaling | 14651849|18439900|36732624|37079666 |
| Hgene | PRKAA1 | GO:1905691 | lipid droplet disassembly | 34077757 |
| Hgene | PRKAA1 | GO:1990044 | protein localization to lipid droplet | 34077757 |
| Tgene | NIPBL | GO:0000122 | negative regulation of transcription by RNA polymerase II | 18854353 |
| Tgene | NIPBL | GO:0006338 | chromatin remodeling | 18854353 |
| Tgene | NIPBL | GO:0007064 | mitotic sister chromatid cohesion | 22628566 |
 Kinase Fusion gene breakpoints across PRKAA1 (5'-gene) Kinase Fusion gene breakpoints across PRKAA1 (5'-gene)* Click on the image to open the UCSC genome browser with custom track showing this image in a new window. |
 Kinase Fusion gene breakpoints across NIPBL (3'-gene) Kinase Fusion gene breakpoints across NIPBL (3'-gene)* Click on the image to open the UCSC genome browser with custom track showing this image in a new window. |
Top |
Kinase Fusion Gene Sample Information |
 Kinase Fusion gene information. Kinase Fusion gene information. |
 Kinase Fusion gene information from four resources (ChiTars 5.0, ChimerDB 4.0, COSMIC, and CCLE) Kinase Fusion gene information from four resources (ChiTars 5.0, ChimerDB 4.0, COSMIC, and CCLE)* All genome coordinats were lifted-over on hg19. * Click on the break point to see the gene structure around the break point region using the UCSC Genome Browser. |
| Source | Sample | Hgene | Hchr | Hbp | Tgene | Tchr | Tbp |
| CCLE | TN-2 | PRKAA1 | chr5 | 40798165 | NIPBL | chr5 | 36953720 |
Top |
Kinase Fusion ORF Analysis |
 Kinase Fusion information from ORFfinder translation from full-length transcript sequence from KinaseFusionDB. Kinase Fusion information from ORFfinder translation from full-length transcript sequence from KinaseFusionDB. |
| Henst | Tenst | Hgene | Hchr | Hbp | Tgene | Tchr | Tbp | Seq length (transcript) | Seq length (amino acids) |
Top |
Kinase Fusion Amino Acid Sequences |
 For individual full-length fusion transcript sequence from KinaseFusionDB, we ran ORFfinder and chose the longest ORF among the all predicted ones. For individual full-length fusion transcript sequence from KinaseFusionDB, we ran ORFfinder and chose the longest ORF among the all predicted ones. |
| >Henst_Tenst_Hgene_Hchr_Hbp_Tgene_Tchr_Tbp_length(fusion AA)_AAseq |
Multiple Sequence Alignment of All Fusion Protein Isoforms |
Top |
Kinase Fusion Protein Functional Features |
 Four levels of functional features of fusion genes Four levels of functional features of fusion genesGo to FGviewer search page for the most frequent breakpoint (https://ccsmweb.uth.edu/FGviewer/:/:) - FGviewer provides the online visualization of the retention search of the protein functional features across DNA, RNA, protein, and pathological levels. - How to search 1. Put your fusion gene symbol. 2. Press the tab key until there will be shown the breakpoint information filled. 4. Go down and press 'Search' tab twice. 4. Go down to have the hyperlink of the search result. 5. Click the hyperlink. 6. See the FGviewer result for your fusion gene. |
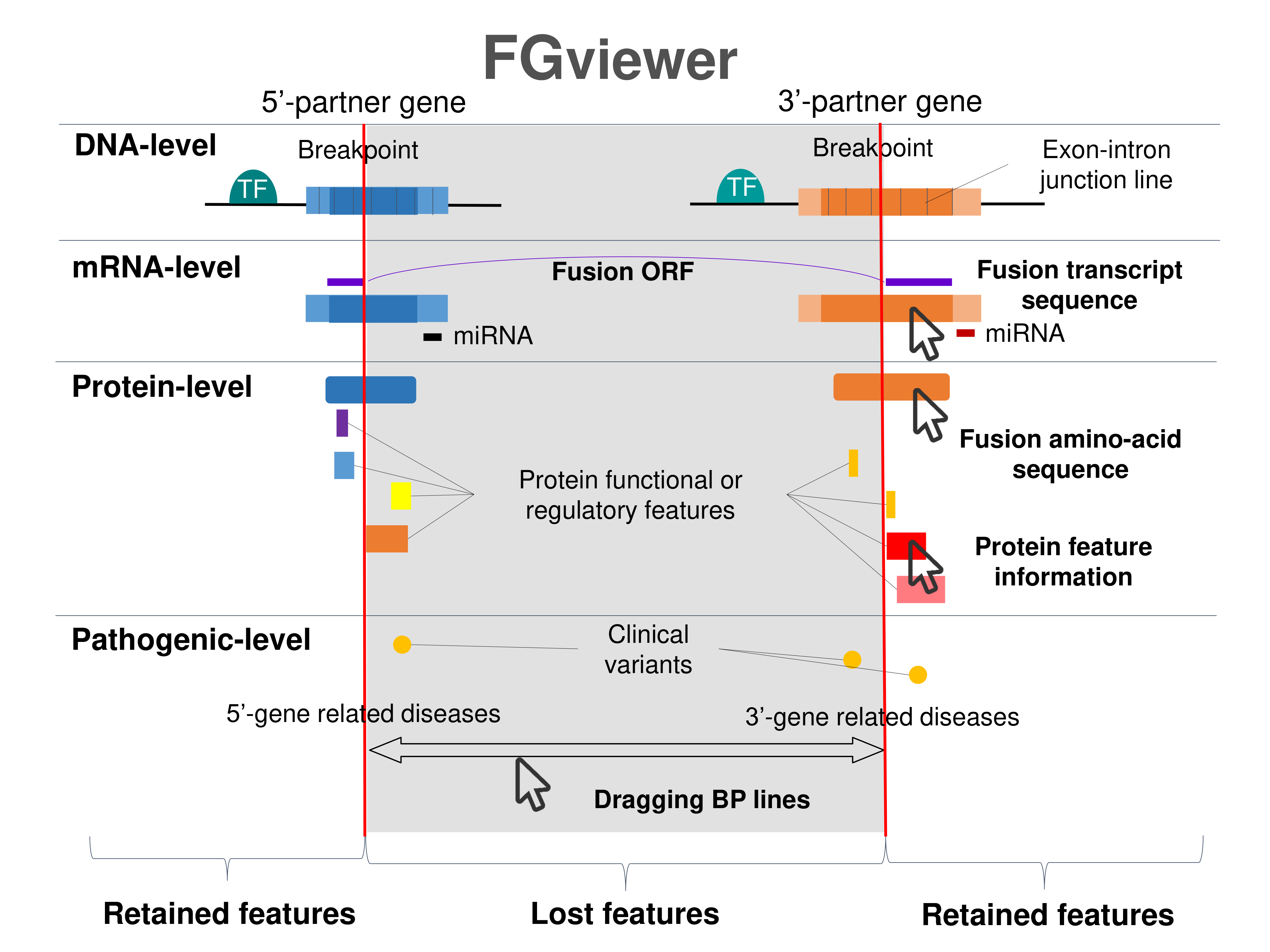 |
 Main function of each fusion partner protein. (from UniProt) Main function of each fusion partner protein. (from UniProt) |
| Hgene | Tgene |
| PRKAA1 | NIPBL |
| FUNCTION: Catalytic subunit of AMP-activated protein kinase (AMPK), an energy sensor protein kinase that plays a key role in regulating cellular energy metabolism (PubMed:17307971, PubMed:17712357, PubMed:24563466, PubMed:37821951). In response to reduction of intracellular ATP levels, AMPK activates energy-producing pathways and inhibits energy-consuming processes: inhibits protein, carbohydrate and lipid biosynthesis, as well as cell growth and proliferation (PubMed:17307971, PubMed:17712357). AMPK acts via direct phosphorylation of metabolic enzymes, and by longer-term effects via phosphorylation of transcription regulators (PubMed:17307971, PubMed:17712357). Regulates lipid synthesis by phosphorylating and inactivating lipid metabolic enzymes such as ACACA, ACACB, GYS1, HMGCR and LIPE; regulates fatty acid and cholesterol synthesis by phosphorylating acetyl-CoA carboxylase (ACACA and ACACB) and hormone-sensitive lipase (LIPE) enzymes, respectively (By similarity). Promotes lipolysis of lipid droplets by mediating phosphorylation of isoform 1 of CHKA (CHKalpha2) (PubMed:34077757). Regulates insulin-signaling and glycolysis by phosphorylating IRS1, PFKFB2 and PFKFB3 (By similarity). AMPK stimulates glucose uptake in muscle by increasing the translocation of the glucose transporter SLC2A4/GLUT4 to the plasma membrane, possibly by mediating phosphorylation of TBC1D4/AS160 (By similarity). Regulates transcription and chromatin structure by phosphorylating transcription regulators involved in energy metabolism such as CRTC2/TORC2, FOXO3, histone H2B, HDAC5, MEF2C, MLXIPL/ChREBP, EP300, HNF4A, p53/TP53, SREBF1, SREBF2 and PPARGC1A (PubMed:11554766, PubMed:11518699, PubMed:15866171, PubMed:17711846, PubMed:18184930). Acts as a key regulator of glucose homeostasis in liver by phosphorylating CRTC2/TORC2, leading to CRTC2/TORC2 sequestration in the cytoplasm (By similarity). In response to stress, phosphorylates 'Ser-36' of histone H2B (H2BS36ph), leading to promote transcription (By similarity). Acts as a key regulator of cell growth and proliferation by phosphorylating FNIP1, TSC2, RPTOR, WDR24 and ATG1/ULK1: in response to nutrient limitation, negatively regulates the mTORC1 complex by phosphorylating RPTOR component of the mTORC1 complex and by phosphorylating and activating TSC2 (PubMed:14651849, PubMed:18439900, PubMed:20160076, PubMed:21205641). Also phosphorylates and inhibits GATOR2 subunit WDR24 in response to nutrient limitation, leading to suppress glucose-mediated mTORC1 activation (PubMed:36732624). In response to energetic stress, phosphorylates FNIP1, inactivating the non-canonical mTORC1 signaling, thereby promoting nuclear translocation of TFEB and TFE3, and inducing transcription of lysosomal or autophagy genes (PubMed:37079666). In response to nutrient limitation, promotes autophagy by phosphorylating and activating ATG1/ULK1 (PubMed:21205641). In that process also activates WDR45/WIPI4 (PubMed:28561066). Phosphorylates CASP6, thereby preventing its autoprocessing and subsequent activation (PubMed:32029622). In response to nutrient limitation, phosphorylates transcription factor FOXO3 promoting FOXO3 mitochondrial import (By similarity). Also acts as a regulator of cellular polarity by remodeling the actin cytoskeleton; probably by indirectly activating myosin (PubMed:17486097). AMPK also acts as a regulator of circadian rhythm by mediating phosphorylation of CRY1, leading to destabilize it (By similarity). May regulate the Wnt signaling pathway by phosphorylating CTNNB1, leading to stabilize it (By similarity). Also has tau-protein kinase activity: in response to amyloid beta A4 protein (APP) exposure, activated by CAMKK2, leading to phosphorylation of MAPT/TAU; however the relevance of such data remains unclear in vivo (By similarity). Also phosphorylates CFTR, EEF2K, KLC1, NOS3 and SLC12A1 (PubMed:20074060, PubMed:12519745). Regulates hepatic lipogenesis. Activated via SIRT3, represses sterol regulatory element-binding protein (SREBP) transcriptional activities and ATP-consuming lipogenesis to restore cellular energy balance. {ECO:0000250|UniProtKB:P54645, ECO:0000250|UniProtKB:Q5EG47, ECO:0000269|PubMed:11518699, ECO:0000269|PubMed:11554766, ECO:0000269|PubMed:12519745, ECO:0000269|PubMed:14651849, ECO:0000269|PubMed:15866171, ECO:0000269|PubMed:17486097, ECO:0000269|PubMed:17711846, ECO:0000269|PubMed:18184930, ECO:0000269|PubMed:18439900, ECO:0000269|PubMed:20074060, ECO:0000269|PubMed:20160076, ECO:0000269|PubMed:21205641, ECO:0000269|PubMed:24563466, ECO:0000269|PubMed:28561066, ECO:0000269|PubMed:32029622, ECO:0000269|PubMed:34077757, ECO:0000269|PubMed:36732624, ECO:0000269|PubMed:37079666, ECO:0000269|PubMed:37821951, ECO:0000303|PubMed:17307971, ECO:0000303|PubMed:17712357}. | FUNCTION: Plays an important role in the loading of the cohesin complex on to DNA. Forms a heterodimeric complex (also known as cohesin loading complex) with MAU2/SCC4 which mediates the loading of the cohesin complex onto chromatin (PubMed:22628566, PubMed:28914604). Plays a role in cohesin loading at sites of DNA damage. Its recruitment to double-strand breaks (DSBs) sites occurs in a CBX3-, RNF8- and RNF168-dependent manner whereas its recruitment to UV irradiation-induced DNA damage sites occurs in a ATM-, ATR-, RNF8- and RNF168-dependent manner (PubMed:28167679). Along with ZNF609, promotes cortical neuron migration during brain development by regulating the transcription of crucial genes in this process. Preferentially binds promoters containing paused RNA polymerase II. Up-regulates the expression of SEMA3A, NRP1, PLXND1 and GABBR2 genes, among others (By similarity). {ECO:0000250|UniProtKB:Q6KCD5, ECO:0000269|PubMed:22628566, ECO:0000269|PubMed:28167679, ECO:0000269|PubMed:28914604}. |
 Retention analysis result of each fusion partner protein across 39 protein features of UniProt such as six molecule processing features, 13 region features, four site features, six amino acid modification features, two natural variation features, five experimental info features, and 3 secondary structure features. Here, because of limited space for viewing, we only show the protein feature retention information belong to the 13 regional features. All retention annotation result can be downloaded at * Minus value of BPloci means that the break pointn is located before the CDS. Retention analysis result of each fusion partner protein across 39 protein features of UniProt such as six molecule processing features, 13 region features, four site features, six amino acid modification features, two natural variation features, five experimental info features, and 3 secondary structure features. Here, because of limited space for viewing, we only show the protein feature retention information belong to the 13 regional features. All retention annotation result can be downloaded at * Minus value of BPloci means that the break pointn is located before the CDS. |
 - Retained domain in the 5'-partner of fusion protein (protein functional feature from UniProt). - Retained domain in the 5'-partner of fusion protein (protein functional feature from UniProt). |
| Partner | Hgeneene | Hbp | Tgeneene | Tbp | ENST | BPexon | TotalExon | Protein feature loci | BPloci | TotalLen | Feature | Note |
 - Retained domain in the 3'-partner of fusion protein (protein functional feature from UniProt). - Retained domain in the 3'-partner of fusion protein (protein functional feature from UniProt). |
| Partner | Hgeneene | Hbp | Tgeneene | Tbp | ENST | BPexon | TotalExon | Protein feature loci | BPloci | TotalLen | Feature | Note |
Top |
Kinase-Substrate Information of PRKAA1_NIPBL |
 Phosphorylation target of the kinase Phosphorylation target of the kinase(phosphosite, 03-17-2024) |
| Kinase | Kinase UniProt Acc | Kinase species | Substrate | Substrate UniProt Acc | Substrate phosphorylated residues | Substrate phosphorylated sites (+/-7AA) | Domain |
| PRKAA1 | Q13131 | human | PRKAB1 | Q9Y478 | S108 | skLPLTRsHNNFVAI | AMPK1_CBM |
| PRKAA1 | Q13131 | human | MAPT | P10636-8 | T231 | KKVAVVRtPPKsPss | |
| PRKAA1 | Q13131 | human | ALDH2 | P05091 | T356 | GNPFDSktEQGPQVD | Aldedh |
| PRKAA1 | Q13131 | human | ATG9A | Q7Z3C6 | S761 | QsIPRsAsYPCAAPR | |
| PRKAA1 | Q13131 | human | CCNY | Q8ND76 | S326 | SARKRsAsADNLtLP | |
| PRKAA1 | Q13131 | human | DBI | P07108 | S21 | RHLkTkPsDEEMLFI | |
| PRKAA1 | Q13131 | human | NET1 | Q7Z628 | S100 | RPLARVTsLANLIsP | |
| PRKAA1 | Q13131 | human | IRS1 | P35568 | S794 | QHLRLSTsSGRLLyA | |
| PRKAA1 | Q13131 | human | FH | P07954 | S75 | yGAQTVRsTMNFkIG | Lyase_1 |
| PRKAA1 | Q13131 | human | CD274 | Q9NZQ7 | S283 | DTNsKkQsDtHLEEt | |
| PRKAA1 | Q13131 | human | PRKN | O60260 | S9 | IVFVRFNssHGFPVE | ubiquitin |
| PRKAA1 | Q13131 | human | AMOTL1 | Q8IY63 | S793 | SSLRPARsVPsIAAA | Angiomotin_C |
| PRKAA1 | Q13131 | human | PRKAA1 | Q13131 | S494 | GtAtPQRsGsVsNyR | AdenylateSensor |
| PRKAA1 | Q13131 | human | SNX17 | Q15036 | S437 | VkLSskLsAVsLRGI | |
| PRKAA1 | Q13131 | human | HDAC5 | Q9UQL6 | S498 | RPLSRtQsSPLPQsP | |
| PRKAA1 | Q13131 | human | HIPK2 | Q9H2X6 | S121 | LMRRStVsLLDTYQK | |
| PRKAA1 | Q13131 | human | PTS | Q03393 | T58 | HNYKVVVtVHGEIDP | PTPS |
| PRKAA1 | Q13131 | human | MCU | Q8NE86 | S57 | TVHQRIAsWQNLGAV | |
| PRKAA1 | Q13131 | human | GAPDH | P04406 | S122 | GAkRVIIsAPsADAP | |
| PRKAA1 | Q13131 | human | GFPT1 | Q06210-2 | S243 | CNLsRVDsttCLFPV | |
| PRKAA1 | Q13131 | human | DNM1L | O00429 | S637 | VPVARKLsAREQRDC | |
| PRKAA1 | Q13131 | human | NOS3 | P29474 | S1177 | TSRIRtQsFsLQERQ | |
| PRKAA1 | Q13131 | human | VASP | P50552 | T278 | LARRRKAtQVGEktP | |
| PRKAA1 | Q13131 | human | ACACA | Q13085 | S80 | LHIRssMsGLHLVkQ | |
| PRKAA1 | Q13131 | human | PRKAA1 | Q13131 | T388 | EtPRARHtLDELNPQ | |
| PRKAA1 | Q13131 | human | PPP1R3C | Q9UQK1 | S293 | LEStIFGsPRLASGL | |
| PRKAA1 | Q13131 | human | TP53BP1 | Q12888 | S1317 | SSLHRtssGtsLSAM | |
| PRKAA1 | Q13131 | human | JAK1 | P23458 | S518 | GSDRsFPsLGDLMsH | |
| PRKAA1 | Q13131 | human | YAP1 | P46937 | S61 | IVHVRGDsEtDLEAL | |
| PRKAA1 | Q13131 | human | SRSF1 | Q07955 | S133 | SGLPPSGsWQDLkDH | RRM_1 |
| PRKAA1 | Q13131 | human | BTRC | Q9Y297 | S82 | SLRQTYNsCARLCLN | |
| PRKAA1 | Q13131 | human | RACK1 | P63244 | T50 | WkLtrDEtNyGIPQR | |
| PRKAA1 | Q13131 | human | PRKAB1 | Q9Y478 | S174 | MVDsQKCsDVsELss | |
| PRKAA1 | Q13131 | human | EPM2A | O95278 | S25 | PELLVVGsRPELGRW | CBM_20 |
| PRKAA1 | Q13131 | human | NFE2L2 | Q16236 | S374 | GDtLLGLsDsEVEEL | |
| PRKAA1 | Q13131 | human | PRKAB1 | Q9Y478 | T158 | NIIQVKktDFEVFDA | AMPK1_CBM |
| PRKAA1 | Q13131 | human | RUNX2 | Q13950 | S118 | AELVRTDsPNFLCSV | Runt |
| PRKAA1 | Q13131 | human | RRN3 | Q9NYV6 | S635 | DTHFRsPssSVGsPP | |
| PRKAA1 | Q13131 | human | KLC2 | Q9H0B6 | S545 | GsLRRsGsFGKLRDA | |
| PRKAA1 | Q13131 | human | HIPK2 | Q9H2X6 | T119 | HNLMRRStVsLLDTY | |
| PRKAA1 | Q13131 | human | PARP1 | P09874 | S177 | LGFRPEYsAsQLkGF | zf-PARP |
| PRKAA1 | Q13131 | human | YAP1 | P46937 | S94 | RLRkLPDsFFkPPEP | |
| PRKAA1 | Q13131 | human | NFE2L2 | Q16236 | S408 | GDMVQPLsPSQGQST | |
| PRKAA1 | Q13131 | human | KPNA2 | P52292 | S105 | QAARkLLsREkQPPI | |
| PRKAA1 | Q13131 | human | PDHA1 | P08559 | S314 | IQEVRSksDPIMLLk | E1_dh |
| PRKAA1 | Q13131 | human | MAPT | P10636-8 | S214 | GsRsRtPsLPtPPtR | |
| PRKAA1 | Q13131 | human | EEF2K | O00418 | S398 | DsLPssPssAtPHSQ | |
| PRKAA1 | Q13131 | human | SMPD3 | Q9NY59 | S209 | GsIKRTAsVEyKGDG | |
| PRKAA1 | Q13131 | human | PFKFB2 | O60825 | S466 | PVRMRRNsFtPLssS | |
| PRKAA1 | Q13131 | human | CFTR | P13569 | S737 | EPLERRLsLVPDSEQ | CFTR_R |
| PRKAA1 | Q13131 | human | EIF2AK3 | Q9NZJ5 | S715 | HIEIIAPsPQRsRSF | |
| PRKAA1 | Q13131 | human | STIM1 | Q13586 | S521 | RDLtHsDsEssLHMs | |
| PRKAA1 | Q13131 | human | MAPT | P10636-8 | S356 | rVQskIGsLDNItHV | Tubulin-binding |
| PRKAA1 | Q13131 | human | NOS3 | P29474 | S633 | WRRKRKEssNtDsAG | Flavodoxin_1 |
| PRKAA1 | Q13131 | human | KCNA5 | P22460 | S592 | KCNVKAKsNVDLRRS | |
| PRKAA1 | Q13131 | human | FOXO3 | O43524 | S626 | SLECDMEsIIRSELM | FOXO-TAD |
| PRKAA1 | Q13131 | human | CDKN1B | P46527 | T198 | PGLRRRQt_______ | |
| PRKAA1 | Q13131 | human | EXO1 | Q9UQ84 | S746 | PGLYkSSsADsLStT | |
| PRKAA1 | Q13131 | human | GLI1 | P08151 | S408 | GPLPRAPsISTVEPk | |
| PRKAA1 | Q13131 | human | DDIT3 | P35638 | S30 | EDLQEVLssDENGGT | |
| PRKAA1 | Q13131 | human | RBBP7 | Q16576 | S314 | LkLHTFEsHkDEIFQ | WD40 |
| PRKAA1 | Q13131 | human | PRPS2 | P11908 | S180 | GGAkRVTsIADrLNV | |
| PRKAA1 | Q13131 | human | PPP1R12C | Q9BZL4 | S452 | AGLQRsAsssWLEGt | |
| PRKAA1 | Q13131 | human | STBD1 | O95210 | S175 | CFAEKLPssNLLKNR | |
| PRKAA1 | Q13131 | human | PAQR3 | Q6TCH7 | T32 | PRGIRLYtyEQIPGS | |
| PRKAA1 | Q13131 | human | KLC2 | Q9H0B6 | S582 | PRMKRAssLNFLNKs | |
| PRKAA1 | Q13131 | human | TP53 | P04637 | T18 | EPPLsQEtFsDLWkL | P53_TAD |
| PRKAA1 | Q13131 | human | EGFR | P00533 | T892 | SILHRIytHQSDVWS | PK_Tyr_Ser-Thr |
| PRKAA1 | Q13131 | human | PRKAA1 | Q13131 | S486 | DEItEAksGtAtPQR | AdenylateSensor |
| PRKAA1 | Q13131 | human | GBF1 | Q92538 | T1338 | GKIHRsAtDADVVNs | |
| PRKAA1 | Q13131 | human | MAPT | P10636-8 | S396 | GAEIVyKsPVVsGDt | |
| PRKAA1 | Q13131 | human | UMPS | P11172 | S214 | VAANHNGsPLsIkEA | |
| PRKAA1 | Q13131 | human | TP53 | P04637 | S20 | PLsQEtFsDLWkLLP | P53_TAD |
| PRKAA1 | Q13131 | human | PRPS1 | P60891 | S180 | GGAkRVTsIADrLNV | |
| PRKAA1 | Q13131 | human | TNNI3 | P19429 | S23 | PAPIRRRssNyRAyA | Troponin-I_N |
| PRKAA1 | Q13131 | human | PDHA1 | P08559 | S295 | RyHGHsMsDPGVsyR | E1_dh |
| PRKAA1 | Q13131 | human | STIM1 | Q13586 | S257 | GLHRAEQsLHDLQER | |
| PRKAA1 | Q13131 | human | BECN1 | Q14457 | S93 | ARMMstEsANsFTLI | |
| PRKAA1 | Q13131 | human | EGLN1 | Q9GZT9 | S61 | HKLVCQGsEGALGHG | |
| PRKAA1 | Q13131 | human | RPTOR | Q8N122 | S792 | DKMRRAssYSsLNSL | |
| PRKAA1 | Q13131 | human | PRKAA1 | Q13131 | S496 | AtPQRsGsVsNyRSC | AdenylateSensor |
| PRKAA1 | Q13131 | human | EIF2AK3 | Q9NZJ5 | S856 | SEATLsIsPPRPTTL | |
| PRKAA1 | Q13131 | human | SIRT1 | Q96EB6 | T344 | GkLLRNYtQNIDTLE | SIR2 |
| PRKAA1 | Q13131 | human | SLC12A1 | Q13621 | S130 | GPKVNRPsLLEIHEQ | AA_permease_N |
| PRKAA1 | Q13131 | human | PIAS1 | O75925 | S510 | sPVsRtPsLPAVDTS | |
| PRKAA1 | Q13131 | human | MAPT | P10636-8 | S262 | NVKskIGstENLkHQ | Tubulin-binding |
| PRKAA1 | Q13131 | human | PPP2R5C | Q13362 | S298 | KYWPKTHsPKEVMFL | B56 |
| PRKAA1 | Q13131 | human | HNF4A | P41235 | S313 | GkIkRLRsQVQVsLE | Hormone_recep |
| PRKAA1 | Q13131 | human | PRKAA1 | Q13131 | T183 | sDGEFLRtsCGsPNy | Pkinase |
| PRKAA1 | Q13131 | human | CSNK1E | P49674 | S389 | rGAPANVsssDLtGR | |
| PRKAA1 | Q13131 | human | ERBB2 | P04626 | T900 | SILRRRFtHQSDVWS | PK_Tyr_Ser-Thr |
| PRKAA1 | Q13131 | human | USP10 | Q14694 | S76 | DtLPRtPsySISstL | |
| PRKAA1 | Q13131 | human | KCNA5 | P22460 | S559 | VQRKVsGsRGSFCKA | |
| PRKAA1 | Q13131 | human | MKNK1 | Q9BUB5-2 | S353 | QVLQRNSsTMDLTLF | |
| PRKAA1 | Q13131 | human | SIRT7 | Q9NRC8 | T153 | TLTHMSItRLHEQKL | SIR2 |
| PRKAA1 | Q13131 | human | PRKAB1 | Q9Y478 | T80 | APAQARPtVFRWTGG | AMPK1_CBM |
| PRKAA1 | Q13131 | human | FANCA | O15360 | S347 | VQMQREWsFARtHPL | Fanconi_A_N |
| PRKAA1 | Q13131 | human | BECN1 | Q14457 | T388 | EEVEKGEtRFCLPYR | APG6 |
| PRKAA1 | Q13131 | human | RASAL2 | Q9UJF2-2 | S351 | TLFARTTsKTKADNI | C2 |
| PRKAA1 | Q13131 | human | GSDME | O60443 | T6 | __MFAkAtRNFLREV | Gasdermin |
| PRKAA1 | Q13131 | human | KIF4A | O95239 | S801 | KLRRRtFsLTEVRGQ | |
| PRKAA1 | Q13131 | human | ULK1 | O75385 | S638 | FDFPKtPssQNLLAL | |
| PRKAA1 | Q13131 | human | FOXO3 | O43524 | S555 | RALsNsVsNMGLSES | |
| PRKAA1 | Q13131 | human | NCL | P19338 | S328 | PELktGIsDVFAkND | RRM_1 |
| PRKAA1 | Q13131 | human | PPP1R3C | Q9UQK1 | S33 | MRLCLAHsPPVKSFL | |
| PRKAA1 | Q13131 | human | PRKAB1 | Q9Y478 | S177 | sQKCsDVsELsssPP | |
| PRKAA1 | Q13131 | human | ZNF692 | Q9BU19 | S470 | VAAHRSKsHPALLLA | |
| PRKAA1 | Q13131 | human | HIPK2 | Q9H2X6 | T1114 | APAALGStGTVAHLV | |
| PRKAA1 | Q13131 | human | CHKA | P35790 | S279 | kKLHKLLsYNLPLEL | Choline_kinase |
| PRKAA1 | Q13131 | human | TP73 | O15350 | S426 | GGMNKLPsVNQLVGQ | |
| PRKAA1 | Q13131 | human | TSC2 | P49815 | S1387 | QPLsKssssPELQtL | |
| PRKAA1 | Q13131 | human | LIPE | Q05469 | S855 | EPMRRsVsEAALAQP | |
| PRKAA1 | Q13131 | human | FOXO3 | O43524 | T179 | SSPDKRLtLSQIyEW | Forkhead |
| PRKAA1 | Q13131 | human | FOXO3 | O43524 | S588 | QTLSDSLsGSSLYST | |
| PRKAA1 | Q13131 | human | FOXO3 | O43524 | S30 | EPQsRPRsCtWPLQR | |
| PRKAA1 | Q13131 | human | BECN1 | Q14457 | S96 | MstEsANsFTLIGEA | |
| PRKAA1 | Q13131 | human | PPP2R5C | Q13362 | S336 | RQLAkCVsSPHFQVA | B56 |
| PRKAA1 | Q13131 | human | FOXO3 | O43524 | S413 | GLMQRSSsFPYTTKG | |
| PRKAA1 | Q13131 | human | GFPT1 | Q06210 | S261 | CNLsRVDsttCLFPV | |
| PRKAA1 | Q13131 | human | TET2 | Q6N021 | S99 | GGIKRtVsEPsLSGL | |
| PRKAA1 | Q13131 | human | EZH2 | Q15910 | T311 | NtYkRKNtETALDNk | |
| PRKAA1 | Q13131 | human | SREBF1 | P36956-3 | S372 | TAVHKSKsLKDLVSA | |
| PRKAA1 | Q13131 | human | SCFD1 | Q8WVM8 | S316 | PKRKNKksYDLTPVD | Sec1 |
| PRKAA1 | Q13131 | human | GSR | P00390 | T507 | TkADFDNtVAIHPts | Pyr_redox_dim |
| PRKAA1 | Q13131 | human | ULK1 | O75385 | S556 | GLGCRLHsAPNLsDL | |
| PRKAA1 | Q13131 | human | EP300 | Q09472 | S89 | SELLRSGsSPNLNMG | |
| PRKAA1 | Q13131 | human | CFTR | P13569 | S768 | LQARRRQsVLNLMtH | CFTR_R |
| PRKAA1 | Q13131 | human | TBC1D1 | Q86TI0 | T596 | AFRRRANtLsHFPIE | |
| PRKAA1 | Q13131 | human | FOXO3 | O43524 | S399 | DNItLPPsQPsPTGG | |
| PRKAA1 | Q13131 | human | ZDHHC13 | Q8IUH4 | S208 | FLLKFNPsLNVVDkI | |
| PRKAA1 | Q13131 | human | TXNIP | Q9H3M7 | S308 | GLssRTssMASRTsS | |
| PRKAA1 | Q13131 | human | HDAC5 | Q9UQL6 | S259 | FPLRkTAsEPNLKVR | |
| PRKAA1 | Q13131 | human | KLC1 | Q07866 | S521 | ENMEkRRsREsLNVD | |
| PRKAA1 | Q13131 | human | PRKAB1 | Q9Y478 | S24 | HKtPRRDssGGtKDG | |
| PRKAA1 | Q13131 | human | ULK1 | O75385 | S317 | SHLASPPsLGEMQQL | |
| PRKAA1 | Q13131 | human | DNMT1 | P26358-2 | S730 | DNIPEMPsPKKMHQG | |
| PRKAA1 | Q13131 | human | GAPVD1 | Q14C86 | S902 | ErLVRsRssDIVSsV | |
| PRKAA1 | Q13131 | human | HSF1 | Q00613 | S121 | NIkRkVtsVSTLksE | |
| PRKAA1 | Q13131 | human | PFKFB3 | Q16875 | S461 | NPLMRRNsVtPLAsP | |
| PRKAA1 | Q13131 | human | NAMPT | P43490 | S314 | PLIIRPDsGNPLDTV | NAPRTase |
| PRKAA1 | Q13131 | human | PRKAA1 | Q13131 | S360 | LAtsPPDsFLDDHHL | |
| PRKAA1 | Q13131 | human | CGN | Q9P2M7 | S137 | GKLLRsHsQAsLAGP | |
| PRKAA1 | Q13131 | human | SIRT2 | Q8IXJ6 | T101 | PDFRSPStGLyDNLE | SIR2 |
| PRKAA1 | Q13131 | human | POU2F1 | P14859 | S335 | RFEALNLsFKNMCKL | Pou |
| PRKAA1 | Q13131 | human | CTBP1 | Q13363 | S158 | REGTRVQsVEQIREV | 2-Hacid_dh_C; 2-Hacid_dh |
| PRKAA1 | Q13131 | human | SKP2 | Q13309 | S256 | QTLLSSCsRLDELNL | |
| PRKAA1 | Q13131 | human | SLC12A2 | P55011 | S77 | RPLGPtPsQsRFQVD | |
| PRKAA1 | Q13131 | human | TBC1D1 | Q86TI0 | S237 | RPMRKsFsQPGLRSL | |
| PRKAA1 | Q13131 | human | CTTN | Q14247 | T401 | QARAKtQtPPVsPAP | |
| PRKAA1 | Q13131 | human | SCFD1 | Q8WVM8 | S303 | EssGVENsPAGARPK | Sec1 |
| PRKAA1 | Q13131 | human | SLC12A2 | P55011 | S242 | GEkLLRPsLAELHDE | AA_permease_N |
| PRKAA1 | Q13131 | human | CD274 | Q9NZQ7 | S195 | EKLFNVtsTLRINTT | C2-set_2 |
| PRKAA1 | Q13131 | human | BRAF | P15056 | S729 | PKIHRSAsEPsLNRA | |
| PRKAA1 | Q13131 | human | NFE2L2 | Q16236 | S433 | PEKELPVsPGHrktP | |
| PRKAA1 | Q13131 | human | MAPT | P10636-8 | S422 | GsIDMVDsPQLAtLA | |
| PRKAA1 | Q13131 | human | EGLN1 | Q9GZT9 | S136 | AAAGGQGsAVAAEAE | |
| PRKAA1 | Q13131 | human | SCN5A | Q14524 | T101 | IVLNKGKtIFRFSAT | |
| PRKAA1 | Q13131 | human | PRKAB1 | Q9Y478 | T148 | IVTsQLGtVNNIIQV | AMPK1_CBM |
| PRKAA1 | Q13131 | human | TNNI3 | P19429 | S150 | tLRrVrIsADAMMQA | Troponin |
| PRKAA1 | Q13131 | human | HAT1 | O14929 | S190 | MWFIETAsFIDVDDE | |
| PRKAA1 | Q13131 | human | CFTR | P13569 | S813 | DIYsRRLsQEtGLEI | CFTR_R |
| PRKAA1 | Q13131 | human | FOXO1 | Q12778 | T649 | PHSVKTttHsWVsG_ | |
| PRKAA1 | Q13131 | human | BECN1 | Q14457 | S90 | IPPARMMstEsANsF | |
| PRKAA1 | Q13131 | human | NEDD4L | Q96PU5 | S795 | VDLKPNGsEIMVTNE | HECT |
| PRKAA1 | Q13131 | human | INSIG1 | O15503 | T222 | ItIAFLAtLITQFLV | TM; INSIG |
| PRKAA1 | Q13131 | human | RPTOR | Q8N122 | S722 | PrLrsVssyGNIRAV | |
| PRKAA1 | Q13131 | human | FOXO1 | Q12778 | S22 | EPLPRPRsCtWPLPR | |
| PRKAA1 | Q13131 | human | SYK | P43405 | S178 | GKISREEsEQIVLIG | SH2 |
| PRKAA1 | Q13131 | human | ACSS2 | Q9NR19 | S659 | PGLPKTRsGKIMRRV | AMP-binding_C |
| PRKAA1 | Q13131 | human | TNNI3 | P19429 | S24 | APIRRRssNyRAyAt | Troponin-I_N |
| PRKAA1 | Q13131 | human | CCAR2 | Q8N163 | S808 | ALVsHNGsLINVGsL | |
| PRKAA1 | Q13131 | human | JAK1 | P23458 | S515 | SLHGSDRsFPsLGDL | |
| PRKAA1 | Q13131 | human | ACACB | O00763 | S222 | PtMRPsMsGLHLVKR | |
| PRKAA1 | Q13131 | human | SQSTM1 | Q13501 | S294 | ssCCsDPskPGGNVE |
 Biological Network Integration of This Kinase and Substrates Biological Network Integration of This Kinase and Substrates (GeneMANIA website) |
 Enriched GO biological processes of the phosphorylation target genes of the kinase Enriched GO biological processes of the phosphorylation target genes of the kinase |
| Kinase | GOID | GO term | P.adjust |
| PRKAA1 | ID | Description | 0.00e+00 |
| PRKAA1 | GO:0062197 | cellular response to chemical stress | 1.11e-14 |
| PRKAA1 | GO:0010506 | regulation of autophagy | 6.81e-14 |
| PRKAA1 | GO:0034599 | cellular response to oxidative stress | 1.56e-13 |
| PRKAA1 | GO:0006979 | response to oxidative stress | 5.34e-12 |
| PRKAA1 | GO:0000302 | response to reactive oxygen species | 2.04e-09 |
| PRKAA1 | GO:1903008 | organelle disassembly | 4.28e-09 |
| PRKAA1 | GO:0071241 | cellular response to inorganic substance | 6.34e-09 |
| PRKAA1 | GO:0034614 | cellular response to reactive oxygen species | 6.34e-09 |
| PRKAA1 | GO:0071248 | cellular response to metal ion | 1.13e-08 |
| PRKAA1 | GO:0031667 | response to nutrient levels | 1.16e-08 |
| PRKAA1 | GO:0062012 | regulation of small molecule metabolic process | 1.17e-08 |
| PRKAA1 | GO:0048511 | rhythmic process | 1.51e-08 |
| PRKAA1 | GO:0010038 | response to metal ion | 2.65e-08 |
| PRKAA1 | GO:0097193 | intrinsic apoptotic signaling pathway | 3.91e-08 |
| PRKAA1 | GO:0006109 | regulation of carbohydrate metabolic process | 4.17e-08 |
| PRKAA1 | GO:2001242 | regulation of intrinsic apoptotic signaling pathway | 4.46e-08 |
| PRKAA1 | GO:0019318 | hexose metabolic process | 4.58e-08 |
| PRKAA1 | GO:0006006 | glucose metabolic process | 4.81e-08 |
| PRKAA1 | GO:0070482 | response to oxygen levels | 8.35e-08 |
| PRKAA1 | GO:0007623 | circadian rhythm | 8.60e-08 |
| PRKAA1 | GO:0000422 | autophagy of mitochondrion | 8.67e-08 |
| PRKAA1 | GO:0061726 | mitochondrion disassembly | 8.67e-08 |
| PRKAA1 | GO:0031669 | cellular response to nutrient levels | 8.99e-08 |
| PRKAA1 | GO:0042542 | response to hydrogen peroxide | 1.07e-07 |
| PRKAA1 | GO:0005996 | monosaccharide metabolic process | 1.38e-07 |
| PRKAA1 | GO:0042594 | response to starvation | 1.49e-07 |
| PRKAA1 | GO:0036293 | response to decreased oxygen levels | 1.61e-07 |
| PRKAA1 | GO:1901653 | cellular response to peptide | 1.98e-07 |
| PRKAA1 | GO:0031668 | cellular response to extracellular stimulus | 2.99e-07 |
| PRKAA1 | GO:0010508 | positive regulation of autophagy | 2.99e-07 |
| PRKAA1 | GO:0006476 | protein deacetylation | 3.00e-07 |
| PRKAA1 | GO:0010821 | regulation of mitochondrion organization | 3.22e-07 |
| PRKAA1 | GO:2001233 | regulation of apoptotic signaling pathway | 4.10e-07 |
| PRKAA1 | GO:0001666 | response to hypoxia | 5.58e-07 |
| PRKAA1 | GO:1903829 | positive regulation of protein localization | 7.14e-07 |
| PRKAA1 | GO:0001558 | regulation of cell growth | 7.27e-07 |
| PRKAA1 | GO:0035601 | protein deacylation | 7.95e-07 |
| PRKAA1 | GO:0070301 | cellular response to hydrogen peroxide | 7.95e-07 |
| PRKAA1 | GO:0071375 | cellular response to peptide hormone stimulus | 8.03e-07 |
| PRKAA1 | GO:0043434 | response to peptide hormone | 1.01e-06 |
| PRKAA1 | GO:0071453 | cellular response to oxygen levels | 1.02e-06 |
| PRKAA1 | GO:0098732 | macromolecule deacylation | 1.02e-06 |
| PRKAA1 | GO:0010906 | regulation of glucose metabolic process | 1.30e-06 |
| PRKAA1 | GO:0009267 | cellular response to starvation | 1.30e-06 |
| PRKAA1 | GO:0042149 | cellular response to glucose starvation | 1.89e-06 |
| PRKAA1 | GO:0071496 | cellular response to external stimulus | 2.71e-06 |
| PRKAA1 | GO:0036294 | cellular response to decreased oxygen levels | 4.22e-06 |
| PRKAA1 | GO:0032869 | cellular response to insulin stimulus | 5.05e-06 |
| PRKAA1 | GO:0016049 | cell growth | 6.12e-06 |
Top |
Related Drugs to PRKAA1_NIPBL |
 Drugs used for this fusion-positive patient. Drugs used for this fusion-positive patient. (Manual curation of PubMed, 04-30-2022 + MyCancerGenome) |
| Hgene | Tgene | Drug | Source | PMID |
 Distribution of the number of studies mentioning PRKAA1-NIPBL and kinase inhibitors the PubMed Abstract (04-01-2024) Distribution of the number of studies mentioning PRKAA1-NIPBL and kinase inhibitors the PubMed Abstract (04-01-2024) |
| Fusion gene - drug pair 1 | Fusion gene - drug pair 2 | PMID | Publication date | DOI | Study title |
Top |
Related Diseases to PRKAA1_NIPBL |
 Diseases that have this fusion gene. Diseases that have this fusion gene. (Manual curation of PubMed, 04-30-2022 + MyCancerGenome) |
| Hgene | Tgene | Disease | Source | PMID |
 Related diseases from the literature mentioned this fusion gene and drug. Related diseases from the literature mentioned this fusion gene and drug. (PubMed, 04-01-2024) |
| MeSH ID | MeSH term |
 Diseases associated with fusion partners. Diseases associated with fusion partners. (DisGeNet 4.0) |
| Partner | Gene | Disease ID | Disease name | # pubmeds | Source |
Top |
Clinical Trials of the Found Drugs/Small Molecules |
 Statistics of the Clinical Trials of the Found Kinase Inibitors from clinicaltrials.gov (06-17-2024) Statistics of the Clinical Trials of the Found Kinase Inibitors from clinicaltrials.gov (06-17-2024) |
 Clinical Trials from clinicaltrials.gov (06-17-2024) Clinical Trials from clinicaltrials.gov (06-17-2024) |
| Fusion Gene | Kinase Inhibitor | NCT ID | Study Status | Phases | Disease | # Enrolment | Date |

