| UTHEALTH HOME ABOUT SBMI A-Z WEBMAIL INSIDE THE UNIVERSITY |

|
|||||||
|
Kinase Fusion Gene:PRKDC_CEP290 |
Kinase Fusion Protein Summary |
 Kinase Fusion gene summary Kinase Fusion gene summary |
| Kinase Fusion partner gene information | Kinase Fusion gene name: PRKDC_CEP290 | KinaseFusionDB ID: KFG5024 | FusionGDB2.0 ID: KFG5024 | Hgene | Tgene | Gene symbol | PRKDC | CEP290 | Gene ID | 5591 | 80184 | |
| Gene name | protein kinase, DNA-activated, catalytic subunit | centrosomal protein 290 | ||||||||||
| Synonyms | DNA-PKC|DNA-PKcs|DNAPK|DNAPKc|DNPK1|HYRC|HYRC1|IMD26|XRCC7|p350 | 3H11Ag|BBS14|CT87|JBTS5|LCA10|MKS4|NPHP6|POC3|SLSN6|rd16 | ||||||||||
| Cytomap | 8q11.21 | 12q21.32 | ||||||||||
| Type of gene | protein-coding | protein-coding | ||||||||||
| Description | DNA-dependent protein kinase catalytic subunitDNA-PK catalytic subunithyper-radiosensitivity of murine scid mutation, complementing 1p460protein kinase, DNA-activated, catalytic polypeptide | centrosomal protein of 290 kDaBardet-Biedl syndrome 14 proteinCTCL tumor antigen se2-2Meckel syndrome, type 4POC3 centriolar protein homologcancer/testis antigen 87centrosomal protein 290kDamonoclonal antibody 3H11 antigennephrocytsin-6prostate c | ||||||||||
| Modification date | 20240411 | 20240403 | ||||||||||
| UniProtAcc | P78527 | O15078 | ||||||||||
| Ensembl transtripts involved in fusion gene | ENST ids | ENST00000314191, ENST00000338368, ENST00000523565, | ENST00000309041, ENST00000397838, ENST00000547691, ENST00000552810, | |||||||||
| Context (manual curation of fusion genes in KinaseFusionDB) | PubMed: PRKDC [Title/Abstract] AND CEP290 [Title/Abstract] AND fusion [Title/Abstract] | |||||||||||
| Most frequent breakpoint (based on all fusion genes of FusionGDB 2.0) | PRKDC(48744375)-CEP290(88533341), # samples:3 | |||||||||||
 Gene ontology of each fusion partner gene with evidence of Inferred from Direct Assay (IDA) from Entrez Gene ontology of each fusion partner gene with evidence of Inferred from Direct Assay (IDA) from Entrez |
| Partner | Gene | GO ID | GO term | PubMed ID |
| Hgene | PRKDC | GO:0000460 | maturation of 5.8S rRNA | 32103174 |
| Hgene | PRKDC | GO:0002218 | activation of innate immune response | 28712728 |
| Hgene | PRKDC | GO:0006468 | protein phosphorylation | 26237645 |
| Hgene | PRKDC | GO:0006974 | DNA damage response | 26237645|29478807|35460603 |
| Hgene | PRKDC | GO:0018105 | peptidyl-serine phosphorylation | 15194694 |
| Hgene | PRKDC | GO:0018105 | peptidyl-serine phosphorylation | 19303849|32103174 |
| Hgene | PRKDC | GO:0018107 | peptidyl-threonine phosphorylation | 32103174 |
| Hgene | PRKDC | GO:0034462 | small-subunit processome assembly | 32103174 |
| Hgene | PRKDC | GO:0160049 | negative regulation of cGAS/STING signaling pathway | 33273464 |
| Hgene | PRKDC | GO:2001034 | positive regulation of double-strand break repair via nonhomologous end joining | 26237645 |
| Tgene | CEP290 | GO:0045893 | positive regulation of DNA-templated transcription | 16682973 |
| Tgene | CEP290 | GO:0060271 | cilium assembly | 26386044 |
 Kinase Fusion gene breakpoints across PRKDC (5'-gene) Kinase Fusion gene breakpoints across PRKDC (5'-gene)* Click on the image to open the UCSC genome browser with custom track showing this image in a new window. |
 Kinase Fusion gene breakpoints across CEP290 (3'-gene) Kinase Fusion gene breakpoints across CEP290 (3'-gene)* Click on the image to open the UCSC genome browser with custom track showing this image in a new window. |
Top |
Kinase Fusion Gene Sample Information |
 Kinase Fusion gene information. Kinase Fusion gene information. |
 Kinase Fusion gene information from four resources (ChiTars 5.0, ChimerDB 4.0, COSMIC, and CCLE) Kinase Fusion gene information from four resources (ChiTars 5.0, ChimerDB 4.0, COSMIC, and CCLE)* All genome coordinats were lifted-over on hg19. * Click on the break point to see the gene structure around the break point region using the UCSC Genome Browser. |
| Source | Sample | Hgene | Hchr | Hbp | Tgene | Tchr | Tbp |
| ChimerDB4 | TCGA-AN-A0FD-01A | PRKDC | chr8 | 48744375 | CEP290 | chr12 | 88533341 |
Top |
Kinase Fusion ORF Analysis |
 Kinase Fusion information from ORFfinder translation from full-length transcript sequence from KinaseFusionDB. Kinase Fusion information from ORFfinder translation from full-length transcript sequence from KinaseFusionDB. |
| Henst | Tenst | Hgene | Hchr | Hbp | Tgene | Tchr | Tbp | Seq length (transcript) | Seq length (amino acids) |
Top |
Kinase Fusion Amino Acid Sequences |
 For individual full-length fusion transcript sequence from KinaseFusionDB, we ran ORFfinder and chose the longest ORF among the all predicted ones. For individual full-length fusion transcript sequence from KinaseFusionDB, we ran ORFfinder and chose the longest ORF among the all predicted ones. |
| >Henst_Tenst_Hgene_Hchr_Hbp_Tgene_Tchr_Tbp_length(fusion AA)_AAseq |
Multiple Sequence Alignment of All Fusion Protein Isoforms |
Top |
Kinase Fusion Protein Functional Features |
 Four levels of functional features of fusion genes Four levels of functional features of fusion genesGo to FGviewer search page for the most frequent breakpoint (https://ccsmweb.uth.edu/FGviewer/:48744375/:88533341) - FGviewer provides the online visualization of the retention search of the protein functional features across DNA, RNA, protein, and pathological levels. - How to search 1. Put your fusion gene symbol. 2. Press the tab key until there will be shown the breakpoint information filled. 4. Go down and press 'Search' tab twice. 4. Go down to have the hyperlink of the search result. 5. Click the hyperlink. 6. See the FGviewer result for your fusion gene. |
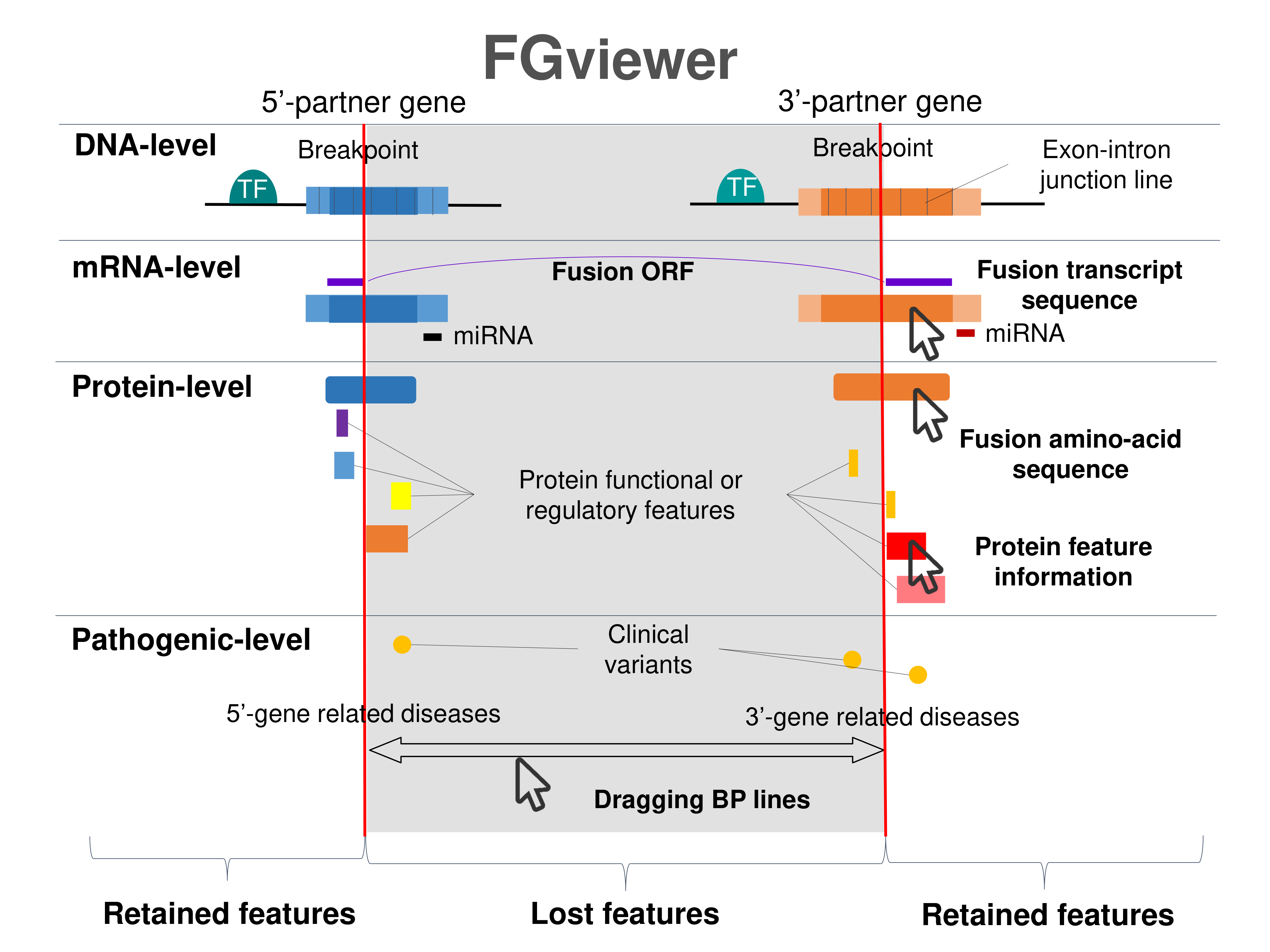 |
 Main function of each fusion partner protein. (from UniProt) Main function of each fusion partner protein. (from UniProt) |
| Hgene | Tgene |
| PRKDC | CEP290 |
| FUNCTION: Serine/threonine-protein kinase that acts as a molecular sensor for DNA damage (PubMed:11955432, PubMed:12649176, PubMed:14734805, PubMed:33854234). Involved in DNA non-homologous end joining (NHEJ) required for double-strand break (DSB) repair and V(D)J recombination (PubMed:11955432, PubMed:12649176, PubMed:14734805, PubMed:33854234, PubMed:34352203). Must be bound to DNA to express its catalytic properties (PubMed:11955432). Promotes processing of hairpin DNA structures in V(D)J recombination by activation of the hairpin endonuclease artemis (DCLRE1C) (PubMed:11955432). Recruited by XRCC5 and XRCC6 to DNA ends and is required to (1) protect and align broken ends of DNA, thereby preventing their degradation, (2) and sequester the DSB for repair by NHEJ (PubMed:15574326, PubMed:11955432, PubMed:12649176, PubMed:14734805, PubMed:33854234). Act as a scaffold protein to aid the localization of DNA repair proteins to the site of damage (PubMed:15574326, PubMed:11955432, PubMed:12649176, PubMed:14734805). The assembly of the DNA-PK complex at DNA ends is also required for the NHEJ ligation step (PubMed:15574326, PubMed:11955432, PubMed:12649176, PubMed:14734805). Found at the ends of chromosomes, suggesting a further role in the maintenance of telomeric stability and the prevention of chromosomal end fusion (By similarity). Also involved in modulation of transcription (PubMed:15574326, PubMed:11955432, PubMed:12649176, PubMed:14734805). As part of the DNA-PK complex, involved in the early steps of ribosome assembly by promoting the processing of precursor rRNA into mature 18S rRNA in the small-subunit processome (PubMed:32103174). Binding to U3 small nucleolar RNA, recruits PRKDC and XRCC5/Ku86 to the small-subunit processome (PubMed:32103174). Recognizes the substrate consensus sequence [ST]-Q (PubMed:15574326, PubMed:11955432, PubMed:12649176, PubMed:14734805). Phosphorylates 'Ser-139' of histone variant H2AX, thereby regulating DNA damage response mechanism (PubMed:14627815, PubMed:16046194). Phosphorylates ASF1A, DCLRE1C, c-Abl/ABL1, histone H1, HSPCA, c-jun/JUN, p53/TP53, PARP1, POU2F1, DHX9, FH, SRF, NHEJ1/XLF, XRCC1, XRCC4, XRCC5, XRCC6, WRN, MYC and RFA2 (PubMed:2507541, PubMed:2247066, PubMed:1597196, PubMed:8407951, PubMed:8464713, PubMed:9362500, PubMed:9139719, PubMed:10026262, PubMed:10467406, PubMed:12509254, PubMed:11889123, PubMed:14612514, PubMed:14599745, PubMed:15177042, PubMed:18644470, PubMed:26666690, PubMed:30247612, PubMed:14704337, PubMed:16397295, PubMed:26237645, PubMed:28712728, PubMed:29478807). Can phosphorylate C1D not only in the presence of linear DNA but also in the presence of supercoiled DNA (PubMed:9679063). Ability to phosphorylate p53/TP53 in the presence of supercoiled DNA is dependent on C1D (PubMed:9363941). Contributes to the determination of the circadian period length by antagonizing phosphorylation of CRY1 'Ser-588' and increasing CRY1 protein stability, most likely through an indirect mechanism (By similarity). Plays a role in the regulation of DNA virus-mediated innate immune response by assembling into the HDP-RNP complex, a complex that serves as a platform for IRF3 phosphorylation and subsequent innate immune response activation through the cGAS-STING pathway (PubMed:28712728). Also regulates the cGAS-STING pathway by catalyzing phosphorylation of CGAS, thereby impairing CGAS oligomerization and activation (PubMed:33273464). Also regulates the cGAS-STING pathway by mediating phosphorylation of PARP1 (PubMed:35460603). {ECO:0000250|UniProtKB:P97313, ECO:0000269|PubMed:10026262, ECO:0000269|PubMed:10467406, ECO:0000269|PubMed:11889123, ECO:0000269|PubMed:11955432, ECO:0000269|PubMed:12509254, ECO:0000269|PubMed:12649176, ECO:0000269|PubMed:14599745, ECO:0000269|PubMed:14612514, ECO:0000269|PubMed:14627815, ECO:0000269|PubMed:14704337, ECO:0000269|PubMed:14734805, ECO:0000269|PubMed:15177042, ECO:0000269|PubMed:15574326, ECO:0000269|PubMed:1597196, ECO:0000269|PubMed:16046194, ECO:0000269|PubMed:16397295, ECO:0000269|PubMed:18644470, ECO:0000269|PubMed:2247066, ECO:0000269|PubMed:2507541, ECO:0000269|PubMed:26237645, ECO:0000269|PubMed:26666690, ECO:0000269|PubMed:28712728, ECO:0000269|PubMed:29478807, ECO:0000269|PubMed:30247612, ECO:0000269|PubMed:32103174, ECO:0000269|PubMed:33273464, ECO:0000269|PubMed:33854234, ECO:0000269|PubMed:34352203, ECO:0000269|PubMed:35460603, ECO:0000269|PubMed:8407951, ECO:0000269|PubMed:8464713, ECO:0000269|PubMed:9139719, ECO:0000269|PubMed:9362500, ECO:0000269|PubMed:9363941, ECO:0000269|PubMed:9679063}. | FUNCTION: Involved in early and late steps in cilia formation. Its association with CCP110 is required for inhibition of primary cilia formation by CCP110 (PubMed:18694559). May play a role in early ciliogenesis in the disappearance of centriolar satellites and in the transition of primary ciliar vesicles (PCVs) to capped ciliary vesicles (CCVs). Required for the centrosomal recruitment of RAB8A and for the targeting of centriole satellite proteins to centrosomes such as of PCM1 (PubMed:24421332). Required for the correct localization of ciliary and phototransduction proteins in retinal photoreceptor cells; may play a role in ciliary transport processes (By similarity). Required for efficient recruitment of RAB8A to primary cilium (PubMed:17705300). In the ciliary transition zone is part of the tectonic-like complex which is required for tissue-specific ciliogenesis and may regulate ciliary membrane composition (By similarity). Involved in regulation of the BBSome complex integrity, specifically for presence of BBS2, BBS5 and BBS8/TTC8 in the complex, and in ciliary targeting of selected BBSome cargos. May play a role in controlling entry of the BBSome complex to cilia possibly implicating IQCB1/NPHP5 (PubMed:25552655). Activates ATF4-mediated transcription (PubMed:16682973). {ECO:0000250|UniProtKB:Q6A078, ECO:0000269|PubMed:16682973, ECO:0000269|PubMed:17705300, ECO:0000269|PubMed:18694559, ECO:0000269|PubMed:24421332, ECO:0000269|PubMed:25552655}. |
 Retention analysis result of each fusion partner protein across 39 protein features of UniProt such as six molecule processing features, 13 region features, four site features, six amino acid modification features, two natural variation features, five experimental info features, and 3 secondary structure features. Here, because of limited space for viewing, we only show the protein feature retention information belong to the 13 regional features. All retention annotation result can be downloaded at * Minus value of BPloci means that the break pointn is located before the CDS. Retention analysis result of each fusion partner protein across 39 protein features of UniProt such as six molecule processing features, 13 region features, four site features, six amino acid modification features, two natural variation features, five experimental info features, and 3 secondary structure features. Here, because of limited space for viewing, we only show the protein feature retention information belong to the 13 regional features. All retention annotation result can be downloaded at * Minus value of BPloci means that the break pointn is located before the CDS. |
 - Retained domain in the 5'-partner of fusion protein (protein functional feature from UniProt). - Retained domain in the 5'-partner of fusion protein (protein functional feature from UniProt). |
| Partner | Hgeneene | Hbp | Tgeneene | Tbp | ENST | BPexon | TotalExon | Protein feature loci | BPloci | TotalLen | Feature | Note |
 - Retained domain in the 3'-partner of fusion protein (protein functional feature from UniProt). - Retained domain in the 3'-partner of fusion protein (protein functional feature from UniProt). |
| Partner | Hgeneene | Hbp | Tgeneene | Tbp | ENST | BPexon | TotalExon | Protein feature loci | BPloci | TotalLen | Feature | Note |
Top |
Kinase-Substrate Information of PRKDC_CEP290 |
 Phosphorylation target of the kinase Phosphorylation target of the kinase(phosphosite, 03-17-2024) |
| Kinase | Kinase UniProt Acc | Kinase species | Substrate | Substrate UniProt Acc | Substrate phosphorylated residues | Substrate phosphorylated sites (+/-7AA) | Domain |
| PRKDC | P78527 | human | POU2F1 | P14859 | S92 | sQPsQQPsVQAAIPQ | |
| PRKDC | P78527 | human | FUS | P35637 | T19 | QSYGAYPtQPGQGYs | |
| PRKDC | P78527 | human | DCLRE1C | Q96SD1 | S503 | NDEITDEsLENFPSS | |
| PRKDC | P78527 | human | MCC | P23508 | S120 | LRsELsQsQHEVNED | |
| PRKDC | P78527 | human | POU5F1 | Q01860 | S93 | PQGGLEtsQPEGEAG | |
| PRKDC | P78527 | human | IRF3 | Q14653 | T135 | GGGSTSDtQEDILDE | |
| PRKDC | P78527 | human | POU2F1 | P14859 | T226 | LQAQNLLtQLPQQsQ | |
| PRKDC | P78527 | human | XRCC4 | Q13426 | S327 | sLEtLRNssPEDLFD | XRCC4 |
| PRKDC | P78527 | human | WRN | Q14191 | S319 | SNNLNLLsFEDSTTG | |
| PRKDC | P78527 | human | XRCC4 | Q13426 | S304 | ENsRPDSsLPETSKK | XRCC4 |
| PRKDC | P78527 | human | TP53 | P04637 | S37 | NVLsPLPsQAMDDLM | TAD2 |
| PRKDC | P78527 | human | POLL | Q9UGP5 | T204 | EASDGEEtQVSAADL | |
| PRKDC | P78527 | human | XPA | P23025 | S196 | RSLEVWGsQEALEEA | |
| PRKDC | P78527 | human | FUS | P35637 | S42 | QQSYSGYsQSTDTSG | |
| PRKDC | P78527 | human | POU2F1 | P14859 | S78 | QSKSNEEsGDsQQPs | |
| PRKDC | P78527 | human | IGFBP3 | P17936 | S183 | KKGHAKDsQRYKVDy | |
| PRKDC | P78527 | human | WRN | Q14191 | S440 | DTsYVIEsDEDLEME | |
| PRKDC | P78527 | human | HSP90AA1 | P07900 | T7 | _MPEEtQtQDQPMEE | |
| PRKDC | P78527 | human | XRCC5 | P13010 | S580 | GAHFsVSsLAEGsVT | |
| PRKDC | P78527 | human | H2AX | P16104 | T136 | PsGGkkAtQAsQEy_ | |
| PRKDC | P78527 | human | POU2F1 | P14859 | T100 | VQAAIPQtQLMLAGG | |
| PRKDC | P78527 | human | HNRNPU | Q00839 | S59 | AMEPGNGsLDLGGDs | |
| PRKDC | P78527 | human | RPA2 | P15927 | S29 | QsPGGFGsPAPsQAE | |
| PRKDC | P78527 | human | TP53 | P04637 | S9 | EEPQsDPsVEPPLsQ | P53_TAD |
| PRKDC | P78527 | human | FH | P07954 | T236 | IkIGRTHtQDAVPLT | Lyase_1 |
| PRKDC | P78527 | human | PNKP | Q96T60 | S114 | EEtRtPEsQPDtPPG | |
| PRKDC | P78527 | human | POU2F1 | P14859 | T162 | ASAATPMtQIPLsQP | |
| PRKDC | P78527 | human | TP53 | P04637 | S46 | AMDDLMLsPDDIEQW | TAD2 |
| PRKDC | P78527 | human | HOXA11 | P31270 | T119 | ANVYHHPtPAVSSNF | DUF3528 |
| PRKDC | P78527 | human | SRF | P11831 | S446 | STMQVSHsQVQEPGG | |
| PRKDC | P78527 | human | TP53 | P04637 | T18 | EPPLsQEtFsDLWkL | P53_TAD |
| PRKDC | P78527 | human | AKT1 | P31749 | S473 | RPHFPQFsysAsGtA | Pkinase_C |
| PRKDC | P78527 | human | XRCC6 | P12956 | S6 | __MsGWEsyykTEGD | |
| PRKDC | P78527 | human | SOX2 | P48431 | S251 | VksEAsssPPVVtSS | |
| PRKDC | P78527 | human | FUS | P35637 | S131 | QPQSGSYsQQPSYGG | |
| PRKDC | P78527 | human | MAPKAP1 | Q9BPZ7 | S186 | VYLPLHssQDRLLPM | CRIM |
| PRKDC | P78527 | human | FUS | P35637 | S30 | QGYsQQSsQPYGQQS | |
| PRKDC | P78527 | human | DCLRE1C | Q96SD1 | S553 | QGsQGWDsQSDTVLL | |
| PRKDC | P78527 | human | GOLPH3 | Q9H4A6 | T148 | KEtQPPEtVQNWIEL | GPP34 |
| PRKDC | P78527 | human | WRN | Q14191 | S467 | DTsYVIEsDEDLEME | |
| PRKDC | P78527 | human | FUS | P35637 | T68 | sQNTGYGtQSTPQGY | |
| PRKDC | P78527 | human | NHEJ1 | Q9H9Q4 | S55 | QVDTSVVsQRAKELN | XLF |
| PRKDC | P78527 | human | POU2F1 | P14859 | S167 | PMtQIPLsQPIQIAQ | |
| PRKDC | P78527 | human | SRF | P11831 | S435 | LTVLNAFsQAPSTMQ | |
| PRKDC | P78527 | human | DCLRE1C | Q96SD1 | S645 | NLSTNADsQsssDFE | |
| PRKDC | P78527 | human | RPA2 | P15927 | S4 | ____MWNsGFEsyGs | |
| PRKDC | P78527 | human | EGR1 | P18146 | S301 | AFATQSGsQDLKALN | |
| PRKDC | P78527 | human | IKBKG | Q9Y6K9 | S43 | PAMLHLPsEQGAPEt | |
| PRKDC | P78527 | human | POU2F1 | P14859 | S85 | sGDsQQPsQPsQQPs | |
| PRKDC | P78527 | human | NABP2 | Q9BQ15 | S134 | NDSNPSAsQPTTGPS | |
| PRKDC | P78527 | human | HNRNPU | Q00839-2 | S59 | AMEPGNGsLDLGGDS | |
| PRKDC | P78527 | human | LIG4 | P49917 | T650 | HLkAPNLtNVNKISN | |
| PRKDC | P78527 | human | VIM | P08670 | S459 | GQVINEtsQHHDDLE | |
| PRKDC | P78527 | human | PRKDC | P78527 | T3950 | GHAFGSAtQFLPVPE | PI3_PI4_kinase |
| PRKDC | P78527 | human | MAPKAP1 | Q9BPZ7 | S367 | DGVFEEDsQIDIATV | |
| PRKDC | P78527 | human | XRCC5 | P13010 | S577 | EQGGAHFsVSsLAEG | |
| PRKDC | P78527 | human | POU2F1 | P14859 | S81 | SNEEsGDsQQPsQPs | |
| PRKDC | P78527 | human | XRCC1 | P18887 | S371 | FANtPKysQVLGLGG | BRCT |
| PRKDC | P78527 | human | PELP1 | Q8IZL8 | S1033 | LAPEALPsQGEVERE | |
| PRKDC | P78527 | human | VIM | P08670 | S430 | LREtNLDsLPLVDtH | |
| PRKDC | P78527 | human | POU2F1 | P14859 | S232 | LtQLPQQsQANLLQS | |
| PRKDC | P78527 | human | FUS | P35637 | T11 | NDYtQQAtQSYGAYP | |
| PRKDC | P78527 | human | XRCC4 | Q13426 | S320 | HISAENMsLEtLRNs | XRCC4 |
| PRKDC | P78527 | human | RAG2 | P55895 | S365 | EQTTFTNsQTSTEDP | RAG2 |
| PRKDC | P78527 | human | XRCC6 | P12956 | S51 | AsKAMFEsQsEDELT | Ku_N |
| PRKDC | P78527 | human | XRCC5 | P13010 | T715 | KDkPsGDtAAVFEEG | |
| PRKDC | P78527 | human | PDX1 | P52945 | T11 | EEQYYAAtQLYKDPC | |
| PRKDC | P78527 | human | PRKDC | P78527 | T2638 | VAGQIRAtQQQHDFt | DNAPKcs_CC5 |
| PRKDC | P78527 | human | TOP1 | P11387 | S10 | GDHLHNDsQIEADFR | |
| PRKDC | P78527 | human | PRKDC | P78527 | T2609 | LtPMFVEtQAsQGtL | DNAPKcs_CC5 |
| PRKDC | P78527 | human | POU2F1 | P14859 | S147 | HsAsQQHsAAGATIS | |
| PRKDC | P78527 | human | FUS | P35637 | S54 | TSGYGQSsYSSYGQs | |
| PRKDC | P78527 | human | XRCC6 | P12956 | S27 | QEENLEAsGDykYsG | |
| PRKDC | P78527 | human | MAPKAP1 | Q9BPZ7 | S343 | DLDSTLEsQSAWEFC | |
| PRKDC | P78527 | human | PNKP | Q96T60 | S126 | PPGtPLVsQDEKRDA | |
| PRKDC | P78527 | human | FUS | P35637 | S142 | SYGGQQQsYGQQQSY | |
| PRKDC | P78527 | human | DCLRE1C | Q96SD1 | S516 | SSTVAGGsQsPKLFS | |
| PRKDC | P78527 | human | NHEJ1 | Q9H9Q4 | T266 | QLVssAPtLsAPEKE | |
| PRKDC | P78527 | human | H1-2 | P16403 | T146 | KkAAGGAtPkKSAKK | |
| PRKDC | P78527 | human | PPARGC1A | Q9UBK2 | S636 | SRRPRyDsYEEYQHE | |
| PRKDC | P78527 | human | CDKN1B | P46527 | S140 | PkTDPSDsQTGLAEQ | |
| PRKDC | P78527 | human | XPA | P23025 | S173 | VKkNPHHsQWGDMKL | XPA_C |
| PRKDC | P78527 | human | POU5F1 | Q01860 | S111 | EsNsDGAsPEPCtVt | |
| PRKDC | P78527 | human | NFKB1 | P19838 | S20 | QMFHLDPsLTHTIFN | |
| PRKDC | P78527 | human | PRKDC | P78527 | T2647 | QQHDFtLtQTADGRs | DNAPKcs_CC5 |
| PRKDC | P78527 | human | DCLRE1C | Q96SD1 | S548 | THITEQGsQGWDsQS | |
| PRKDC | P78527 | human | MCC | P23508 | S118 | SELRsELsQsQHEVN | |
| PRKDC | P78527 | human | PRKDC | P78527 | S2612 | MFVEtQAsQGtLQtR | DNAPKcs_CC5 |
| PRKDC | P78527 | human | YBX1 | P67809 | T89 | EDVFVHQtAIkkNNP | CSD |
| PRKDC | P78527 | human | POLR2A | P24928 | S1616 | TPQSPSysPtsPsYS | RNA_pol_Rpb1_R |
| PRKDC | P78527 | human | MED1 | Q15648 | T1457 | HsKsPAytPQNLDsE | |
| PRKDC | P78527 | human | RPA2 | P15927 | S8 | MWNsGFEsyGsssyG | |
| PRKDC | P78527 | human | ASF1A | Q9Y294 | S192 | GWSTSENsLNVMLES | |
| PRKDC | P78527 | human | HSP90AA1 | P07900 | T5 | ___MPEEtQtQDQPM | |
| PRKDC | P78527 | human | FUS | P35637 | S84 | STGGYGSsQSsQSSY | |
| PRKDC | P78527 | human | AIRE | O43918 | T68 | ALLSWLLtQDSTAIL | HSR |
| PRKDC | P78527 | human | HOXA11 | P31270 | S98 | RDCLQAPsAAGVPGD | DUF3528 |
| PRKDC | P78527 | human | RPA2 | P15927 | S33 | GFGsPAPsQAEkkSR | |
| PRKDC | P78527 | human | TP53 | P04637 | S20 | PLsQEtFsDLWkLLP | P53_TAD |
| PRKDC | P78527 | human | FUS | P35637 | S117 | SGsYGSSsQSSSYGQ | |
| PRKDC | P78527 | human | AIRE | O43918 | S156 | RGTASPGsQLKAKPP | |
| PRKDC | P78527 | human | PRKAG1 | P54619 | T284 | LKCYLHEtLETIINR | CBS |
| PRKDC | P78527 | human | RPA2 | P15927 | S23 | GAGGYtQsPGGFGsP | |
| PRKDC | P78527 | human | NHEJ1 | Q9H9Q4 | S263 | QPEQLVssAPtLsAP | |
| PRKDC | P78527 | human | FUS | P35637 | S61 | sYSSYGQsQNTGYGt | |
| PRKDC | P78527 | human | FUS | P35637 | S26 | tQPGQGYsQQSsQPY | |
| PRKDC | P78527 | human | GOLPH3 | Q9H4A6 | T143 | ALkHVKEtQPPEtVQ | GPP34 |
| PRKDC | P78527 | human | DCLRE1C | Q96SD1 | S538 | HISsQNSsQSTHITE | |
| PRKDC | P78527 | human | XRCC4 | Q13426 | S260 | KDDsIIssLDVtDIA | XRCC4 |
| PRKDC | P78527 | human | POU2F1 | P14859 | S141 | AAAVQQHsAsQQHsA | |
| PRKDC | P78527 | human | XRCC4 | Q13426 | S328 | LEtLRNssPEDLFDE | XRCC4 |
| PRKDC | P78527 | human | PRKDC | P78527 | S3205 | tPLPEDNsMNVDQDG | FAT |
| PRKDC | P78527 | human | POLR2A | P24928 | S1621 | SysPtsPsYSPTSPS | RNA_pol_Rpb1_R |
| PRKDC | P78527 | human | TRIM28 | Q13263 | S824 | LPGAGLssQELsGGP | |
| PRKDC | P78527 | human | CHEK2 | O96017 | T68 | SsLEtVstQELYsIP | |
| PRKDC | P78527 | human | XRCC6 | P12956 | S33 | AsGDykYsGRDsLIF | |
| PRKDC | P78527 | human | CASP2 | P42575 | S139 | LSCDYDLsLPFPVCE | |
| PRKDC | P78527 | human | RPA2 | P15927 | T21 | yGGAGGYtQsPGGFG | |
| PRKDC | P78527 | human | H2AX | P16104 | S139 | GkkAtQAsQEy____ | |
| PRKDC | P78527 | human | TGM2 | P21980 | T162 | ERQEyVLtQQGFIYQ | |
| PRKDC | P78527 | human | PRKAG1 | P54619 | S192 | KPEFMSKsLEELQIG | CBS |
| PRKDC | P78527 | human | FUS | P35637 | S87 | GYGSsQSsQSSYGQQ | |
| PRKDC | P78527 | human | NHEJ1 | Q9H9Q4 | S251 | AsLQGIDsQCVNQPE | |
| PRKDC | P78527 | human | CHEK2 | O96017 | T387 | LMRtLCGtPtyLAPE | Pkinase |
| PRKDC | P78527 | human | POU2F1 | P14859 | S88 | sQQPsQPsQQPsVQA | |
| PRKDC | P78527 | human | TP53 | P04637 | S15 | PsVEPPLsQEtFsDL | P53_TAD |
| PRKDC | P78527 | human | NHEJ1 | Q9H9Q4 | S245 | PHTSNSAsLQGIDsQ | |
| PRKDC | P78527 | human | POU2F1 | P14859 | S143 | AVQQHsAsQQHsAAG | |
| PRKDC | P78527 | human | TDP1 | Q9NUW8 | S81 | PKRQKsGsQEDLGWC | |
| PRKDC | P78527 | human | USF1 | P22415 | S262 | RQSNHRLsEELQGLD | |
| PRKDC | P78527 | human | PARP1 | P09874 | T594 | RSWGRVGtVIGSNkL | WGR |
| PRKDC | P78527 | human | NHEJ1 | Q9H9Q4 | T223 | DLYMAVTtQEVQVGQ | |
| PRKDC | P78527 | human | PRKDC | P78527 | S2056 | VQsYsYSsQDPRPAT | DNAPKcs_CC3 |
| PRKDC | P78527 | human | NHEJ1 | Q9H9Q4 | S132 | LASPSLVsQHLIRPL | XLF |
| PRKDC | P78527 | human | VCP | P55072 | S784 | NQGGAGPsQGsGGGt | |
| PRKDC | P78527 | human | DCLRE1C | Q96SD1 | S534 | GESTHISsQNSsQST | |
| PRKDC | P78527 | human | PNPT1 | Q8TCS8 | S776 | IVMGEPIsQSSSNsQ | |
| PRKDC | P78527 | human | VIM | P08670 | S56 | srsLyAssPGGVyAt | Filament_head |
| PRKDC | P78527 | human | CHEK2 | O96017 | T383 | GEtsLMRtLCGtPty | Pkinase |
| PRKDC | P78527 | human | AKT1 | P31749 | T308 | kDGAtMKtFCGtPEy | Pkinase |
| PRKDC | P78527 | human | FUS | P35637 | T7 | _MASNDYtQQAtQSY | |
| PRKDC | P78527 | human | FUS | P35637 | S112 | APSSTSGsYGSSsQS |
 Biological Network Integration of This Kinase and Substrates Biological Network Integration of This Kinase and Substrates (GeneMANIA website) |
 Enriched GO biological processes of the phosphorylation target genes of the kinase Enriched GO biological processes of the phosphorylation target genes of the kinase |
| Kinase | GOID | GO term | P.adjust |
| PRKDC | ID | Description | 0.00e+00 |
| PRKDC | GO:0006302 | double-strand break repair | 1.34e-18 |
| PRKDC | GO:0010212 | response to ionizing radiation | 3.95e-15 |
| PRKDC | GO:0006310 | DNA recombination | 7.61e-14 |
| PRKDC | GO:0009314 | response to radiation | 1.28e-13 |
| PRKDC | GO:0010332 | response to gamma radiation | 1.34e-13 |
| PRKDC | GO:0006303 | double-strand break repair via nonhomologous end joining | 1.52e-12 |
| PRKDC | GO:0000723 | telomere maintenance | 1.03e-11 |
| PRKDC | GO:0032200 | telomere organization | 7.93e-11 |
| PRKDC | GO:0071480 | cellular response to gamma radiation | 3.10e-10 |
| PRKDC | GO:0051054 | positive regulation of DNA metabolic process | 8.10e-10 |
| PRKDC | GO:0006266 | DNA ligation | 8.67e-10 |
| PRKDC | GO:0033151 | V(D)J recombination | 1.23e-09 |
| PRKDC | GO:0071479 | cellular response to ionizing radiation | 5.50e-09 |
| PRKDC | GO:0006284 | base-excision repair | 8.28e-09 |
| PRKDC | GO:0000725 | recombinational repair | 1.89e-08 |
| PRKDC | GO:0071478 | cellular response to radiation | 1.98e-08 |
| PRKDC | GO:0031571 | mitotic G1 DNA damage checkpoint signaling | 2.83e-08 |
| PRKDC | GO:0044819 | mitotic G1/S transition checkpoint signaling | 2.83e-08 |
| PRKDC | GO:0071214 | cellular response to abiotic stimulus | 2.83e-08 |
| PRKDC | GO:0104004 | cellular response to environmental stimulus | 2.83e-08 |
| PRKDC | GO:0002520 | immune system development | 3.82e-08 |
| PRKDC | GO:0002200 | somatic diversification of immune receptors | 3.36e-07 |
| PRKDC | GO:0071897 | DNA biosynthetic process | 5.73e-07 |
| PRKDC | GO:2000628 | regulation of miRNA metabolic process | 6.14e-07 |
| PRKDC | GO:1902807 | negative regulation of cell cycle G1/S phase transition | 1.19e-06 |
| PRKDC | GO:0010165 | response to X-ray | 1.91e-06 |
| PRKDC | GO:0010586 | miRNA metabolic process | 2.21e-06 |
| PRKDC | GO:0000012 | single strand break repair | 2.73e-06 |
| PRKDC | GO:2000630 | positive regulation of miRNA metabolic process | 2.75e-06 |
| PRKDC | GO:0090398 | cellular senescence | 2.75e-06 |
| PRKDC | GO:0000724 | double-strand break repair via homologous recombination | 3.55e-06 |
| PRKDC | GO:0048732 | gland development | 4.67e-06 |
| PRKDC | GO:0060218 | hematopoietic stem cell differentiation | 4.67e-06 |
| PRKDC | GO:0002562 | somatic diversification of immune receptors via germline recombination within a single locus | 4.67e-06 |
| PRKDC | GO:0016444 | somatic cell DNA recombination | 4.67e-06 |
| PRKDC | GO:0000077 | DNA damage checkpoint signaling | 5.86e-06 |
| PRKDC | GO:0000075 | cell cycle checkpoint signaling | 6.09e-06 |
| PRKDC | GO:0045739 | positive regulation of DNA repair | 6.89e-06 |
| PRKDC | GO:1901988 | negative regulation of cell cycle phase transition | 7.29e-06 |
| PRKDC | GO:0006260 | DNA replication | 7.33e-06 |
| PRKDC | GO:0031570 | DNA integrity checkpoint signaling | 8.27e-06 |
| PRKDC | GO:1903131 | mononuclear cell differentiation | 8.45e-06 |
| PRKDC | GO:0044773 | mitotic DNA damage checkpoint signaling | 8.95e-06 |
| PRKDC | GO:1902806 | regulation of cell cycle G1/S phase transition | 9.77e-06 |
| PRKDC | GO:0006289 | nucleotide-excision repair | 9.77e-06 |
| PRKDC | GO:0006977 | DNA damage respons | 2.22e-07 |
| PRKDC | GO:2000134 | negative regulation of G1/S transition of mitotic cell cycle | 9.77e-06 |
| PRKDC | GO:0007093 | mitotic cell cycle checkpoint signaling | 9.77e-06 |
| PRKDC | GO:0033077 | T cell differentiation in thymus | 1.00e-05 |
Top |
Related Drugs to PRKDC_CEP290 |
 Drugs used for this fusion-positive patient. Drugs used for this fusion-positive patient. (Manual curation of PubMed, 04-30-2022 + MyCancerGenome) |
| Hgene | Tgene | Drug | Source | PMID |
 Distribution of the number of studies mentioning PRKDC-CEP290 and kinase inhibitors the PubMed Abstract (04-01-2024) Distribution of the number of studies mentioning PRKDC-CEP290 and kinase inhibitors the PubMed Abstract (04-01-2024) |
| Fusion gene - drug pair 1 | Fusion gene - drug pair 2 | PMID | Publication date | DOI | Study title |
Top |
Related Diseases to PRKDC_CEP290 |
 Diseases that have this fusion gene. Diseases that have this fusion gene. (Manual curation of PubMed, 04-30-2022 + MyCancerGenome) |
| Hgene | Tgene | Disease | Source | PMID |
 Related diseases from the literature mentioned this fusion gene and drug. Related diseases from the literature mentioned this fusion gene and drug. (PubMed, 04-01-2024) |
| MeSH ID | MeSH term |
 Diseases associated with fusion partners. Diseases associated with fusion partners. (DisGeNet 4.0) |
| Partner | Gene | Disease ID | Disease name | # pubmeds | Source |
Top |
Clinical Trials of the Found Drugs/Small Molecules |
 Statistics of the Clinical Trials of the Found Kinase Inibitors from clinicaltrials.gov (06-17-2024) Statistics of the Clinical Trials of the Found Kinase Inibitors from clinicaltrials.gov (06-17-2024) |
 Clinical Trials from clinicaltrials.gov (06-17-2024) Clinical Trials from clinicaltrials.gov (06-17-2024) |
| Fusion Gene | Kinase Inhibitor | NCT ID | Study Status | Phases | Disease | # Enrolment | Date |

