| UTHEALTH HOME ABOUT SBMI A-Z WEBMAIL INSIDE THE UNIVERSITY |

|
|||||||
|
Kinase Fusion Gene:ROCK1_CABYR |
Kinase Fusion Protein Summary |
 Kinase Fusion gene summary Kinase Fusion gene summary |
| Kinase Fusion partner gene information | Kinase Fusion gene name: ROCK1_CABYR | KinaseFusionDB ID: KFG5418 | FusionGDB2.0 ID: KFG5418 | Hgene | Tgene | Gene symbol | ROCK1 | CABYR | Gene ID | 6093 | 26256 | |
| Gene name | Rho associated coiled-coil containing protein kinase 1 | calcium binding tyrosine phosphorylation regulated | ||||||||||
| Synonyms | P160ROCK|ROCK-I | CABYRa|CABYRc|CABYRc/d|CABYRe|CBP86|CT88|FSP-2|FSP2 | ||||||||||
| Cytomap | 18q11.1 | 18q11.2 | ||||||||||
| Type of gene | protein-coding | protein-coding | ||||||||||
| Description | rho-associated protein kinase 1p160 ROCK-1renal carcinoma antigen NY-REN-35 | calcium-binding tyrosine phosphorylation-regulated proteincalcium binding tyrosine-(Y)-phosphorylation regulated (fibrousheathin 2)calcium-binding protein 86cancer/testis antigen 88fibrousheathin IIfibrousheathin-2testis tissue sperm-binding protein | ||||||||||
| Modification date | 20240305 | 20240411 | ||||||||||
| UniProtAcc | Q13464 | O75952 | ||||||||||
| Ensembl transtripts involved in fusion gene | ENST ids | ENST00000399799, | ENST00000327201, ENST00000399481, ENST00000399499, ENST00000415309, ENST00000581397, ENST00000399496, | |||||||||
| Context (manual curation of fusion genes in KinaseFusionDB) | PubMed: ROCK1 [Title/Abstract] AND CABYR [Title/Abstract] AND fusion [Title/Abstract] | |||||||||||
| Most frequent breakpoint (based on all fusion genes of FusionGDB 2.0) | ROCK1(18690779)-CABYR(21735665), # samples:3 | |||||||||||
 Gene ontology of each fusion partner gene with evidence of Inferred from Direct Assay (IDA) from Entrez Gene ontology of each fusion partner gene with evidence of Inferred from Direct Assay (IDA) from Entrez |
| Partner | Gene | GO ID | GO term | PubMed ID |
| Hgene | ROCK1 | GO:0006468 | protein phosphorylation | 23093407 |
| Hgene | ROCK1 | GO:0007159 | leukocyte cell-cell adhesion | 12082081 |
| Hgene | ROCK1 | GO:0018105 | peptidyl-serine phosphorylation | 18573880 |
| Hgene | ROCK1 | GO:0022614 | membrane to membrane docking | 12082081 |
| Hgene | ROCK1 | GO:0030334 | regulation of cell migration | 23093407 |
| Hgene | ROCK1 | GO:0032091 | negative regulation of protein binding | 18573880 |
| Hgene | ROCK1 | GO:0032956 | regulation of actin cytoskeleton organization | 25911094 |
| Hgene | ROCK1 | GO:0050900 | leukocyte migration | 12082081 |
| Hgene | ROCK1 | GO:0050901 | leukocyte tethering or rolling | 12082081 |
| Hgene | ROCK1 | GO:0070507 | regulation of microtubule cytoskeleton organization | 23093407 |
 Kinase Fusion gene breakpoints across ROCK1 (5'-gene) Kinase Fusion gene breakpoints across ROCK1 (5'-gene)* Click on the image to open the UCSC genome browser with custom track showing this image in a new window. |
 Kinase Fusion gene breakpoints across CABYR (3'-gene) Kinase Fusion gene breakpoints across CABYR (3'-gene)* Click on the image to open the UCSC genome browser with custom track showing this image in a new window. |
Top |
Kinase Fusion Gene Sample Information |
 Kinase Fusion gene information. Kinase Fusion gene information. |
 Kinase Fusion gene information from four resources (ChiTars 5.0, ChimerDB 4.0, COSMIC, and CCLE) Kinase Fusion gene information from four resources (ChiTars 5.0, ChimerDB 4.0, COSMIC, and CCLE)* All genome coordinats were lifted-over on hg19. * Click on the break point to see the gene structure around the break point region using the UCSC Genome Browser. |
| Source | Sample | Hgene | Hchr | Hbp | Tgene | Tchr | Tbp |
| ChimerDB4 | TCGA-97-7552-01A | ROCK1 | chr18 | 18690779 | CABYR | chr18 | 21735665 |
| ChimerDB4 | TCGA-97-7552 | ROCK1 | chr18 | 18690778 | CABYR | chr18 | 21735664 |
Top |
Kinase Fusion ORF Analysis |
 Kinase Fusion information from ORFfinder translation from full-length transcript sequence from KinaseFusionDB. Kinase Fusion information from ORFfinder translation from full-length transcript sequence from KinaseFusionDB. |
| Henst | Tenst | Hgene | Hchr | Hbp | Tgene | Tchr | Tbp | Seq length (transcript) | Seq length (amino acids) |
Top |
Kinase Fusion Amino Acid Sequences |
 For individual full-length fusion transcript sequence from KinaseFusionDB, we ran ORFfinder and chose the longest ORF among the all predicted ones. For individual full-length fusion transcript sequence from KinaseFusionDB, we ran ORFfinder and chose the longest ORF among the all predicted ones. |
| >Henst_Tenst_Hgene_Hchr_Hbp_Tgene_Tchr_Tbp_length(fusion AA)_AAseq |
Multiple Sequence Alignment of All Fusion Protein Isoforms |
Top |
Kinase Fusion Protein Functional Features |
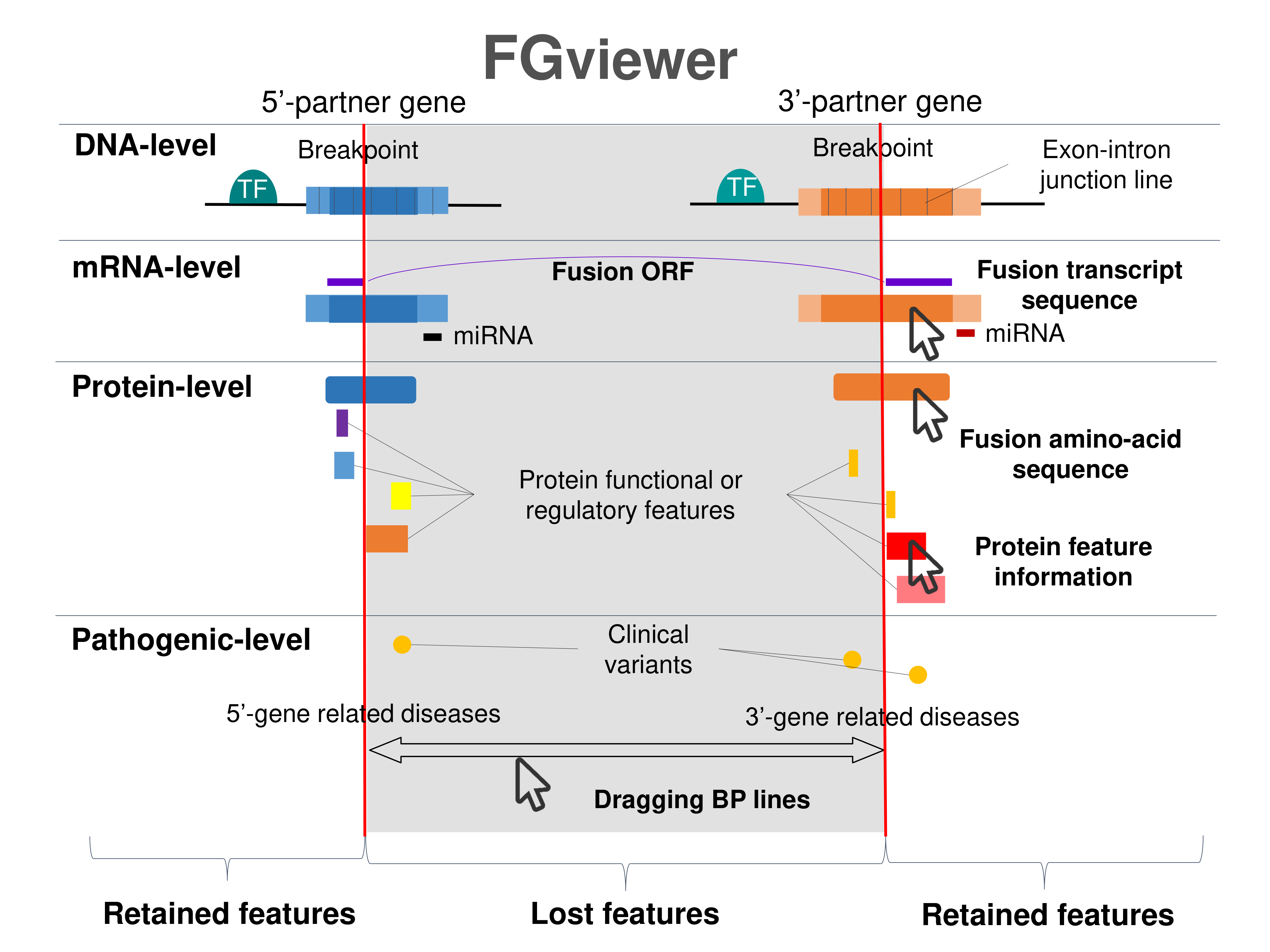
 Four levels of functional features of fusion genes Four levels of functional features of fusion genesGo to FGviewer search page for the most frequent breakpoint (https://ccsmweb.uth.edu/FGviewer/:18690779/:21735665) - FGviewer provides the online visualization of the retention search of the protein functional features across DNA, RNA, protein, and pathological levels. - How to search 1. Put your fusion gene symbol. 2. Press the tab key until there will be shown the breakpoint information filled. 4. Go down and press 'Search' tab twice. 4. Go down to have the hyperlink of the search result. 5. Click the hyperlink. 6. See the FGviewer result for your fusion gene. |
 |
 Main function of each fusion partner protein. (from UniProt) Main function of each fusion partner protein. (from UniProt) |
| Hgene | Tgene |
| ROCK1 | CABYR |
| FUNCTION: Protein kinase which is a key regulator of the actin cytoskeleton and cell polarity (PubMed:10436159, PubMed:10652353, PubMed:11018042, PubMed:11283607, PubMed:17158456, PubMed:18573880, PubMed:19131646, PubMed:8617235, PubMed:9722579). Involved in regulation of smooth muscle contraction, actin cytoskeleton organization, stress fiber and focal adhesion formation, neurite retraction, cell adhesion and motility via phosphorylation of DAPK3, GFAP, LIMK1, LIMK2, MYL9/MLC2, TPPP, PFN1 and PPP1R12A (PubMed:10436159, PubMed:10652353, PubMed:11018042, PubMed:11283607, PubMed:17158456, PubMed:18573880, PubMed:19131646, PubMed:8617235, PubMed:9722579, PubMed:23093407, PubMed:23355470). Phosphorylates FHOD1 and acts synergistically with it to promote SRC-dependent non-apoptotic plasma membrane blebbing (PubMed:18694941). Phosphorylates JIP3 and regulates the recruitment of JNK to JIP3 upon UVB-induced stress (PubMed:19036714). Acts as a suppressor of inflammatory cell migration by regulating PTEN phosphorylation and stability (By similarity). Acts as a negative regulator of VEGF-induced angiogenic endothelial cell activation (PubMed:19181962). Required for centrosome positioning and centrosome-dependent exit from mitosis (By similarity). Plays a role in terminal erythroid differentiation (PubMed:21072057). Inhibits podocyte motility via regulation of actin cytoskeletal dynamics and phosphorylation of CFL1 (By similarity). Promotes keratinocyte terminal differentiation (PubMed:19997641). Involved in osteoblast compaction through the fibronectin fibrillogenesis cell-mediated matrix assembly process, essential for osteoblast mineralization (By similarity). May regulate closure of the eyelids and ventral body wall by inducing the assembly of actomyosin bundles (By similarity). {ECO:0000250|UniProtKB:P70335, ECO:0000250|UniProtKB:Q8MIT6, ECO:0000269|PubMed:10436159, ECO:0000269|PubMed:10652353, ECO:0000269|PubMed:11018042, ECO:0000269|PubMed:11283607, ECO:0000269|PubMed:17158456, ECO:0000269|PubMed:18573880, ECO:0000269|PubMed:18694941, ECO:0000269|PubMed:19036714, ECO:0000269|PubMed:19131646, ECO:0000269|PubMed:19181962, ECO:0000269|PubMed:19997641, ECO:0000269|PubMed:21072057, ECO:0000269|PubMed:23093407, ECO:0000269|PubMed:23355470, ECO:0000269|PubMed:8617235, ECO:0000269|PubMed:9722579}. | FUNCTION: May function as a regulator of both motility- and head-associated functions such as capacitation and the acrosome reaction. Isoform 1 binds calcium in vitro. Isoform 2 and isoform 6 probably bind calcium. Isoform 3 and isoform 5 do not bind calcium in vitro. Isoform 4 probably does not bind calcium. |
 Retention analysis result of each fusion partner protein across 39 protein features of UniProt such as six molecule processing features, 13 region features, four site features, six amino acid modification features, two natural variation features, five experimental info features, and 3 secondary structure features. Here, because of limited space for viewing, we only show the protein feature retention information belong to the 13 regional features. All retention annotation result can be downloaded at * Minus value of BPloci means that the break pointn is located before the CDS. Retention analysis result of each fusion partner protein across 39 protein features of UniProt such as six molecule processing features, 13 region features, four site features, six amino acid modification features, two natural variation features, five experimental info features, and 3 secondary structure features. Here, because of limited space for viewing, we only show the protein feature retention information belong to the 13 regional features. All retention annotation result can be downloaded at * Minus value of BPloci means that the break pointn is located before the CDS. |
 - Retained domain in the 5'-partner of fusion protein (protein functional feature from UniProt). - Retained domain in the 5'-partner of fusion protein (protein functional feature from UniProt). |
| Partner | Hgeneene | Hbp | Tgeneene | Tbp | ENST | BPexon | TotalExon | Protein feature loci | BPloci | TotalLen | Feature | Note |
 - Retained domain in the 3'-partner of fusion protein (protein functional feature from UniProt). - Retained domain in the 3'-partner of fusion protein (protein functional feature from UniProt). |
| Partner | Hgeneene | Hbp | Tgeneene | Tbp | ENST | BPexon | TotalExon | Protein feature loci | BPloci | TotalLen | Feature | Note |
Top |
Kinase-Substrate Information of ROCK1_CABYR |
 Phosphorylation target of the kinase Phosphorylation target of the kinase(phosphosite, 03-17-2024) |
| Kinase | Kinase UniProt Acc | Kinase species | Substrate | Substrate UniProt Acc | Substrate phosphorylated residues | Substrate phosphorylated sites (+/-7AA) | Domain |
| ROCK1 | Q13464 | human | NOS3 | P29474 | T495 | TGITRKKtFKEVANA | |
| ROCK1 | Q13464 | human | RDX | P35241 | T564 | AGRDKyKtLRQIRQG | ERM_C |
| ROCK1 | Q13464 | human | SLC9A1 | P19634 | T653 | LRsYNRHtLVADPyE | NEXCaM_BD |
| ROCK1 | Q13464 | human | ARHGAP35 | Q9NRY4 | T1173 | GRFASYRtsFsVGsD | RhoGAP_pG1_pG2 |
| ROCK1 | Q13464 | human | FHOD1 | Q9Y613 | S1137 | RsRGNRKsLRRtLKs | |
| ROCK1 | Q13464 | human | ARHGAP24 | Q8N264 | S391 | RsSMNNGsPtALSGs | |
| ROCK1 | Q13464 | human | FHOD1 | Q9Y613 | T1141 | NRKsLRRtLKsGLGD | |
| ROCK1 | Q13464 | human | EZR | P15311 | T567 | QGRDkyKtLRQIRQG | ERM_C |
| ROCK1 | Q13464 | human | GFAP | P14136 | S38 | LGPGTRLsLARMPPP | Filament_head |
| ROCK1 | Q13464 | human | PTEN | P60484 | S229 | VkIYSsNsGPtRRED | PTEN_C2 |
| ROCK1 | Q13464 | human | ARHGAP35 | Q9NRY4 | S1236 | PKPKPRPsITKATWE | RhoGAP_pG1_pG2 |
| ROCK1 | Q13464 | human | DES | P17661 | T17 | RVssYRRtFGGAPGF | Filament_head |
| ROCK1 | Q13464 | human | AQP2 | P41181 | T269 | PQsLPRGtkA_____ | |
| ROCK1 | Q13464 | human | FHOD1 | Q9Y613 | S1131 | AARERKRsRGNRKsL | |
| ROCK1 | Q13464 | human | PRMT5 | O14744 | T80 | LsGRDWNtLIVGkLS | PRMT5_TIM |
| ROCK1 | Q13464 | human | RND3 | P61587 | S11 | RRAsQkLsSksIMDP | |
| ROCK1 | Q13464 | human | MYL9 | P24844 | T19 | KKRPQRAtsNVFAMF | |
| ROCK1 | Q13464 | human | DES | P17661 | S60 | VyQVsRtsGGAGGLG | Filament_head |
| ROCK1 | Q13464 | human | KCNK3 | O14649 | S336 | IPRDLStsDTCVEQS | |
| ROCK1 | Q13464 | human | ARHGAP24 | Q8N264 | T452 | GLEKTQTtPNGSLQA | |
| ROCK1 | Q13464 | human | PFN1 | P07737 | S138 | MAsHLRRsQy_____ | Profilin |
| ROCK1 | Q13464 | human | RND3 | P61587 | S218 | QRATKRIsHMPsRPE | |
| ROCK1 | Q13464 | human | H3C1 | P68431 | S10 | tkQtArkstGGkAPr | Histone |
| ROCK1 | Q13464 | human | ARHGAP24 | Q8N264 | S437 | SGIVTNGsFSSSNAE | |
| ROCK1 | Q13464 | human | RND3 | P61587 | S7 | _MKERRAsQkLsSks | |
| ROCK1 | Q13464 | human | LIMK2 | P53671 | T505 | NDRKKRYtVVGNPYW | PK_Tyr_Ser-Thr |
| ROCK1 | Q13464 | human | ARHGAP35 | Q9NRY4 | S1174 | RFASYRtsFsVGsDD | RhoGAP_pG1_pG2 |
| ROCK1 | Q13464 | human | H3C1 | P68431 | S28 | ATkAArksAPATGGV | Histone |
| ROCK1 | Q13464 | human | VIM | P08670 | S72 | ssAVrLrssVPGVRL | Filament_head |
| ROCK1 | Q13464 | human | GFAP | P14136 | S13 | ItsAArrsyVSsGEM | Filament_head |
| ROCK1 | Q13464 | human | DES | P17661 | T76 | LRAsRLGttRtPssy | Filament_head |
| ROCK1 | Q13464 | human | PTK2 | Q05397 | S732 | SsEGFYPsPQHMVQT | |
| ROCK1 | Q13464 | human | PAWR | Q96IZ0 | T163 | LREKRRstGVVNIPA | |
| ROCK1 | Q13464 | human | SOX9 | P48436 | S181 | YQPRRRKsVKNGQAE | |
| ROCK1 | Q13464 | human | PPP1R12A | O14974 | T853 | PREKRRstGVsFWtQ | |
| ROCK1 | Q13464 | human | PPP1R12A | O14974 | T696 | ARQsRRstQGVtLtD | |
| ROCK1 | Q13464 | human | MAPK8IP3 | Q9UPT6 | S365 | RLDRtGssPTQGIVN | |
| ROCK1 | Q13464 | human | SCRIB | Q14160 | S1508 | WRAARMksLEQDALR | |
| ROCK1 | Q13464 | human | MARCKS | P29966 | S159 | kkKKKRFsFkKsFkL | MARCKS |
| ROCK1 | Q13464 | human | RND3 | P61587 | S240 | LRKDKAKsCTVM___ | |
| ROCK1 | Q13464 | human | TPPP | O94811 | S107 | FSKIKGKsCRTITFE | p25-alpha |
| ROCK1 | Q13464 | human | GFAP | P14136 | T7 | _MERRRItsAArrsy | Filament_head |
| ROCK1 | Q13464 | human | ARHGAP35 | Q9NRY4 | S1150 | LERGRKVsIVsKPVL | RhoGAP_pG1_pG2 |
| ROCK1 | Q13464 | human | ARHGAP24 | Q8N264 | S415 | HKLDVsRsPPLMVkk | |
| ROCK1 | Q13464 | human | ARHGAP19 | Q14CB8 | S422 | QkRARsRsFsGLIkR | |
| ROCK1 | Q13464 | human | MYL12B | O14950 | S20 | KRPQRAtsNVFAMFD | |
| ROCK1 | Q13464 | human | DPYSL2 | Q16555 | T555 | DNIPRRttQRIVAPP | |
| ROCK1 | Q13464 | human | TPPP | O94811 | S159 | sGVtKAIssPtVSRL | p25-alpha |
| ROCK1 | Q13464 | human | ARHGAP35 | Q9NRY4 | T1226 | LRsLRRNtKKPKPKP | RhoGAP_pG1_pG2 |
| ROCK1 | Q13464 | human | ADD1 | P35611 | T445 | QkQQREKtRWLNSGR | |
| ROCK1 | Q13464 | human | NR3C1 | P04150 | T519 | SENPGNKtIVPAtLP | |
| ROCK1 | Q13464 | human | MYL9 | P24844 | S20 | KRPQRAtsNVFAMFD | |
| ROCK1 | Q13464 | human | DES | P17661 | T77 | RAsRLGttRtPssyG | Filament_head |
| ROCK1 | Q13464 | human | ADD1 | P35611 | T480 | TKEDGHRtstsAVPN | |
| ROCK1 | Q13464 | human | SCRIB | Q14160 | S1378 | EGPPKRVsLVGADDL | |
| ROCK1 | Q13464 | human | MSN | P26038 | T558 | LGRDKyKtLRQIRQG | ERM_C |
| ROCK1 | Q13464 | human | NR3C1 | P04150 | S617 | WRSYRQSsANLLCFA | Hormone_recep |
| ROCK1 | Q13464 | human | PPP1R12B | O60237 | T646 | ARQtRRstQGVtLtD | |
| ROCK1 | Q13464 | human | ARHGAP24 | Q8N264 | S413 | sVHKLDVsRsPPLMV | |
| ROCK1 | Q13464 | human | DAPK3 | O43293 | T265 | KDPKRRMtIAQSLEH | Pkinase |
| ROCK1 | Q13464 | human | MYL12B | O14950 | T19 | KKRPQRAtsNVFAMF | |
| ROCK1 | Q13464 | human | ARHGAP24 | Q8N264 | S402 | LSGsKTNsPKNsVHK | |
| ROCK1 | Q13464 | human | DES | P17661 | S12 | ysSSQRVssYRRtFG | Filament_head |
| ROCK1 | Q13464 | human | LIMK1 | P53667 | T508 | PDRKKRYtVVGNPYW | PK_Tyr_Ser-Thr |
| ROCK1 | Q13464 | human | DAPK3 | O43293 | T299 | PERRRLKtTRLkEyt | |
| ROCK1 | Q13464 | human | MAPK8IP3 | Q9UPT6 | S314 | RAREKRDsRNMEVQV | |
| ROCK1 | Q13464 | human | TPPP | O94811 | S32 | DRAAKRLsLEsEGAG | |
| ROCK1 | Q13464 | human | MAPK8IP3 | Q9UPT6 | S364 | TRLDRtGssPTQGIV | |
| ROCK1 | Q13464 | human | PTEN | P60484 | T232 | YSsNsGPtRREDKFM | PTEN_C2 |
| ROCK1 | Q13464 | human | KCNK3 | O14649 | S393 | GLMKRRssV______ | |
| ROCK1 | Q13464 | human | PPP1R12A | O14974 | S852 | RPREKRRstGVsFWt |
 Biological Network Integration of This Kinase and Substrates Biological Network Integration of This Kinase and Substrates (GeneMANIA website) |
 Enriched GO biological processes of the phosphorylation target genes of the kinase Enriched GO biological processes of the phosphorylation target genes of the kinase |
| Kinase | GOID | GO term | P.adjust |
| ROCK1 | ID | Description | 0.00e+00 |
| ROCK1 | GO:0051017 | actin filament bundle assembly | 1.38e-08 |
| ROCK1 | GO:0061572 | actin filament bundle organization | 1.38e-08 |
| ROCK1 | GO:0007015 | actin filament organization | 1.55e-08 |
| ROCK1 | GO:1902946 | protein localization to early endosome | 2.04e-06 |
| ROCK1 | GO:0008360 | regulation of cell shape | 2.21e-06 |
| ROCK1 | GO:0032956 | regulation of actin cytoskeleton organization | 3.56e-05 |
| ROCK1 | GO:0036010 | protein localization to endosome | 3.56e-05 |
| ROCK1 | GO:0042063 | gliogenesis | 3.56e-05 |
| ROCK1 | GO:0022604 | regulation of cell morphogenesis | 5.66e-05 |
| ROCK1 | GO:0032535 | regulation of cellular component size | 5.66e-05 |
| ROCK1 | GO:0010001 | glial cell differentiation | 5.66e-05 |
| ROCK1 | GO:0032970 | regulation of actin filament-based process | 5.66e-05 |
| ROCK1 | GO:1902903 | regulation of supramolecular fiber organization | 6.36e-05 |
| ROCK1 | GO:0110053 | regulation of actin filament organization | 7.54e-05 |
| ROCK1 | GO:0030953 | astral microtubule organization | 7.54e-05 |
| ROCK1 | GO:1905668 | positive regulation of protein localization to endosome | 1.29e-04 |
| ROCK1 | GO:0032231 | regulation of actin filament bundle assembly | 1.49e-04 |
| ROCK1 | GO:1905666 | regulation of protein localization to endosome | 1.49e-04 |
| ROCK1 | GO:0061028 | establishment of endothelial barrier | 1.75e-04 |
| ROCK1 | GO:1903651 | positive regulation of cytoplasmic transport | 2.07e-04 |
| ROCK1 | GO:0002064 | epithelial cell development | 2.07e-04 |
| ROCK1 | GO:2000641 | regulation of early endosome to late endosome transport | 3.81e-04 |
| ROCK1 | GO:0051893 | regulation of focal adhesion assembly | 3.81e-04 |
| ROCK1 | GO:0090109 | regulation of cell-substrate junction assembly | 3.81e-04 |
| ROCK1 | GO:0001885 | endothelial cell development | 3.90e-04 |
| ROCK1 | GO:0150116 | regulation of cell-substrate junction organization | 4.78e-04 |
| ROCK1 | GO:0043254 | regulation of protein-containing complex assembly | 6.69e-04 |
| ROCK1 | GO:0042060 | wound healing | 6.86e-04 |
| ROCK1 | GO:0048041 | focal adhesion assembly | 1.04e-03 |
| ROCK1 | GO:0048708 | astrocyte differentiation | 1.10e-03 |
| ROCK1 | GO:0008361 | regulation of cell size | 1.28e-03 |
| ROCK1 | GO:1900027 | regulation of ruffle assembly | 1.29e-03 |
| ROCK1 | GO:0051492 | regulation of stress fiber assembly | 1.30e-03 |
| ROCK1 | GO:0031345 | negative regulation of cell projection organization | 1.30e-03 |
| ROCK1 | GO:1903649 | regulation of cytoplasmic transport | 1.30e-03 |
| ROCK1 | GO:0007044 | cell-substrate junction assembly | 1.30e-03 |
| ROCK1 | GO:0120032 | regulation of plasma membrane bounded cell projection assembly | 1.59e-03 |
| ROCK1 | GO:0060491 | regulation of cell projection assembly | 1.59e-03 |
| ROCK1 | GO:0150115 | cell-substrate junction organization | 1.59e-03 |
| ROCK1 | GO:0031032 | actomyosin structure organization | 1.64e-03 |
| ROCK1 | GO:0110020 | regulation of actomyosin structure organization | 1.64e-03 |
| ROCK1 | GO:0044319 | wound healin | 4.74e-05 |
| ROCK1 | GO:0048709 | oligodendrocyte differentiation | 1.64e-03 |
| ROCK1 | GO:0090504 | epiboly | 1.70e-03 |
| ROCK1 | GO:1902115 | regulation of organelle assembly | 1.73e-03 |
| ROCK1 | GO:0030038 | contractile actin filament bundle assembly | 1.73e-03 |
| ROCK1 | GO:0043149 | stress fiber assembly | 1.73e-03 |
| ROCK1 | GO:0007163 | establishment or maintenance of cell polarity | 2.08e-03 |
| ROCK1 | GO:0045022 | early endosome to late endosome transport | 2.34e-03 |
Top |
Related Drugs to ROCK1_CABYR |
 Drugs used for this fusion-positive patient. Drugs used for this fusion-positive patient. (Manual curation of PubMed, 04-30-2022 + MyCancerGenome) |
| Hgene | Tgene | Drug | Source | PMID |
 Distribution of the number of studies mentioning ROCK1-CABYR and kinase inhibitors the PubMed Abstract (04-01-2024) Distribution of the number of studies mentioning ROCK1-CABYR and kinase inhibitors the PubMed Abstract (04-01-2024) |
| Fusion gene - drug pair 1 | Fusion gene - drug pair 2 | PMID | Publication date | DOI | Study title |
Top |
Related Diseases to ROCK1_CABYR |
 Diseases that have this fusion gene. Diseases that have this fusion gene. (Manual curation of PubMed, 04-30-2022 + MyCancerGenome) |
| Hgene | Tgene | Disease | Source | PMID |
 Related diseases from the literature mentioned this fusion gene and drug. Related diseases from the literature mentioned this fusion gene and drug. (PubMed, 04-01-2024) |
| MeSH ID | MeSH term |
 Diseases associated with fusion partners. Diseases associated with fusion partners. (DisGeNet 4.0) |
| Partner | Gene | Disease ID | Disease name | # pubmeds | Source |
Top |
Clinical Trials of the Found Drugs/Small Molecules |
 Statistics of the Clinical Trials of the Found Kinase Inibitors from clinicaltrials.gov (06-17-2024) Statistics of the Clinical Trials of the Found Kinase Inibitors from clinicaltrials.gov (06-17-2024) |
 Clinical Trials from clinicaltrials.gov (06-17-2024) Clinical Trials from clinicaltrials.gov (06-17-2024) |
| Fusion Gene | Kinase Inhibitor | NCT ID | Study Status | Phases | Disease | # Enrolment | Date |

