| UTHEALTH HOME ABOUT SBMI A-Z WEBMAIL INSIDE THE UNIVERSITY |

|
|||||||
|
Kinase Fusion Gene:TRIP11_PDGFRB |
Kinase Fusion Protein Summary |
 Kinase Fusion gene summary Kinase Fusion gene summary |
| Kinase Fusion partner gene information | Kinase Fusion gene name: TRIP11_PDGFRB | KinaseFusionDB ID: KFG6822 | FusionGDB2.0 ID: KFG6822 | Hgene | Tgene | Gene symbol | TRIP11 | PDGFRB | Gene ID | 9321 | 5159 | |
| Gene name | thyroid hormone receptor interactor 11 | platelet derived growth factor receptor beta | ||||||||||
| Synonyms | ACG1A|CEV14|GMAP-210|GMAP210|ODCD|ODCD1|TRIP-11|TRIP230 | CD140B|IBGC4|IMF1|JTK12|KOGS|PDGFR|PDGFR-1|PDGFR1|PENTT | ||||||||||
| Cytomap | 14q32.12 | 5q32 | ||||||||||
| Type of gene | protein-coding | protein-coding | ||||||||||
| Description | thyroid receptor-interacting protein 11Golgi-microtubule-associated protein of 210 kDaTR-interacting protein 11clonal evolution-related gene on chromosome 14 proteingolgi-associated microtubule-binding protein 210 | platelet-derived growth factor receptor betaActivated tyrosine kinase PDGFRBCD140 antigen-like family member BNDEL1-PDGFRBPDGF-R-betaPDGFR-betabeta-type platelet-derived growth factor receptorplatelet-derived growth factor receptor 1platelet-deriv | ||||||||||
| Modification date | 20240411 | 20240413 | ||||||||||
| UniProtAcc | Q15643 | P09619 | ||||||||||
| Ensembl transtripts involved in fusion gene | ENST ids | ENST00000267622, ENST00000555105, | ENST00000523456, ENST00000261799, | |||||||||
| Context (manual curation of fusion genes in KinaseFusionDB) | PubMed: TRIP11 [Title/Abstract] AND PDGFRB [Title/Abstract] AND fusion [Title/Abstract] | |||||||||||
| Most frequent breakpoint (based on all fusion genes of FusionGDB 2.0) | ||||||||||||
 Gene ontology of each fusion partner gene with evidence of Inferred from Direct Assay (IDA) from Entrez Gene ontology of each fusion partner gene with evidence of Inferred from Direct Assay (IDA) from Entrez |
| Partner | Gene | GO ID | GO term | PubMed ID |
| Tgene | PDGFRB | GO:0007165 | signal transduction | 10821867 |
| Tgene | PDGFRB | GO:0010863 | positive regulation of phospholipase C activity | 1653029 |
| Tgene | PDGFRB | GO:0018108 | peptidyl-tyrosine phosphorylation | 1653029|2536956|2850496 |
| Tgene | PDGFRB | GO:0030335 | positive regulation of cell migration | 17470632 |
| Tgene | PDGFRB | GO:0032516 | positive regulation of phosphoprotein phosphatase activity | 7691811 |
| Tgene | PDGFRB | GO:0038091 | positive regulation of cell proliferation by VEGF-activated platelet derived growth factor receptor signaling pathway | 17470632 |
| Tgene | PDGFRB | GO:0046777 | protein autophosphorylation | 2536956|2850496 |
| Tgene | PDGFRB | GO:0048008 | platelet-derived growth factor receptor signaling pathway | 1314164|2536956 |
| Tgene | PDGFRB | GO:0051897 | positive regulation of phosphatidylinositol 3-kinase/protein kinase B signal transduction | 1314164 |
| Tgene | PDGFRB | GO:0060326 | cell chemotaxis | 2554309|17991872 |
 Kinase Fusion gene breakpoints across TRIP11 (5'-gene) Kinase Fusion gene breakpoints across TRIP11 (5'-gene)* Click on the image to open the UCSC genome browser with custom track showing this image in a new window. |
 Kinase Fusion gene breakpoints across PDGFRB (3'-gene) Kinase Fusion gene breakpoints across PDGFRB (3'-gene)* Click on the image to open the UCSC genome browser with custom track showing this image in a new window. |
Top |
Kinase Fusion Gene Sample Information |
 Kinase Fusion gene information. Kinase Fusion gene information. |
 Kinase Fusion gene information from four resources (ChiTars 5.0, ChimerDB 4.0, COSMIC, and CCLE) Kinase Fusion gene information from four resources (ChiTars 5.0, ChimerDB 4.0, COSMIC, and CCLE)* All genome coordinats were lifted-over on hg19. * Click on the break point to see the gene structure around the break point region using the UCSC Genome Browser. |
| Source | Sample | Hgene | Hchr | Hbp | Tgene | Tchr | Tbp |
| ChimerKB3 | . | TRIP11 | chr14 | 92454627 | PDGFRB | chr5 | 149506177 |
Top |
Kinase Fusion ORF Analysis |
 Kinase Fusion information from ORFfinder translation from full-length transcript sequence from KinaseFusionDB. Kinase Fusion information from ORFfinder translation from full-length transcript sequence from KinaseFusionDB. |
| Henst | Tenst | Hgene | Hchr | Hbp | Tgene | Tchr | Tbp | Seq length (transcript) | Seq length (amino acids) |
| ENST00000267622 | ENST00000261799 | TRIP11 | chr14 | 92454627 | PDGFRB | chr5 | 149506177 | 9302 | 2378 |
Top |
Kinase Fusion Amino Acid Sequences |
 For individual full-length fusion transcript sequence from KinaseFusionDB, we ran ORFfinder and chose the longest ORF among the all predicted ones. For individual full-length fusion transcript sequence from KinaseFusionDB, we ran ORFfinder and chose the longest ORF among the all predicted ones. |
| >Henst_Tenst_Hgene_Hchr_Hbp_Tgene_Tchr_Tbp_length(fusion AA)_AAseq >ENST00000267622_ENST00000261799_TRIP11_chr14_92454627_PDGFRB_chr5_149506177_length(amino acids)=2378 MRTQLCVIVSCLAKAGVQGYLVGSPGGAKRFLFSERTGSFSKLAAMSSWLGGLGSGLGQSLGQVGGSLASLTGQISNFTKDMLMEGTEEV EAELPDSRTKEIEAIHAILRSENERLKKLCTDLEEKHEASEIQIKQQSTSYRNQLQQKEVEISHLKARQIALQDQLLKLQSAAQSVPSGA GVPATTASSSFAYGISHHPSAFHDDDMDFGDIISSQQEINRLSNEVSRLESEVGHWRHIAQTSKAQGTDNSDQSEICKLQNIIKELKQNR SQEIDDHQHEMSVLQNAHQQKLTEISRRHREELSDYEERIEELENLLQQGGSGVIETDLSKIYEMQKTIQVLQIEKVESTKKMEQLEDKI KDINKKLSSAENDRDILRREQEQLNVEKRQIMEECENLKLECSKLQPSAVKQSDTMTEKERILAQSASVEEVFRLQQALSDAENEIMRLS SLNQDNSLAEDNLKLKMRIEVLEKEKSLLSQEKEELQMSLLKLNNEYEVIKSTATRDISLDSELHDLRLNLEAKEQELNQSISEKETLIA EIEELDRQNQEATKHMILIKDQLSKQQNEGDSIISKLKQDLNDEKKRVHQLEDDKMDITKELDVQKEKLIQSEVALNDLHLTKQKLEDKV ENLVDQLNKSQESNVSIQKENLELKEHIRQNEEELSRIRNELMQSLNQDSNSNFKDTLLKEREAEVRNLKQNLSELEQLNENLKKVAFDV KMENEKLVLACEDVRHQLEECLAGNNQLSLEKNTIVETLKMEKGEIEAELCWAKKRLLEEANKYEKTIEELSNARNLNTSALQLEHEHLI KLNQKKDMEIAELKKNIEQMDTDHKETKDVLSSSLEEQKQLTQLINKKEIFIEKLKERSSKLQEELDKYSQALRKNEILRQTIEEKDRSL GSMKEENNHLQEELERLREEQSRTAPVADPKTLDSVTELASEVSQLNTIKEHLEEEIKHHQKIIEDQNQSKMQLLQSLQEQKKEMDEFRY QHEQMNATHTQLFLEKDEEIKSLQKTIEQIKTQLHEERQDIQTDNSDIFQETKVQSLNIENGSEKHDLSKAETERLVKGIKERELEIKLL NEKNISLTKQIDQLSKDEVGKLTQIIQQKDLEIQALHARISSTSHTQDVVYLQQQLQAYAMEREKVFAVLNEKTRENSHLKTEYHKMMDI VAAKEAALIKLQDENKKLSTRFESSGQDMFRETIQNLSRIIREKDIEIDALSQKCQTLLAVLQTSSTGNEAGGVNSNQFEELLQERDKLK QQVKKMEEWKQQVMTTVQNMQHESAQLQEELHQLQAQVLVDSDNNSKLQVDYTGLIQSYEQNETKLKNFGQELAQVQHSIGQLCNTKDLL LGKLDIISPQLSSASLLTPQSAECLRASKSEVLSESSELLQQELEELRKSLQEKDATIRTLQENNHRLSDSIAATSELERKEHEQTDSEI KQLKEKQDVLQKLLKEKDLLIKAKSDQLLSSNENFTNKVNENELLRQAVTNLKERILILEMDIGKLKGENEKIVETYRGKETEYQALQET NMKFSMMLREKEFECHSMKEKALAFEQLLKEKEQGKTGELNQLLNAVKSMQEKTVVFQQERDQVMLALKQKQMENTALQNEVQRLRDKEF RSNQELERLRNHLLESEDSYTREALAAEDREAKLRKKVTVLEEKLVSSSNAMENASHQASVQVESLQEQLNVVSKQRDETALQLSVSQEQ VKQYALSLANLQMVLEHFQQEEKAMYSAELEKQKQLIAEWKKNAENLEGKVISLQECLDEANAALDSASRLTEQLDVKEEQIEELKRQTL PFKVVVISAILALVVLTIISLIILIMLWQKKPRYEIRWKVIESVSSDGHEYIYVDPMQLPYDSTWELPRDQLVLGRTLGSGAFGQVVEAT AHGLSHSQATMKVAVKMLKSTARSSEKQALMSELKIMSHLGPHLNVVNLLGACTKGGPIYIITEYCRYGDLVDYLHRNKHTFLQHHSDKR RPPSAELYSNALPVGLPLPSHVSLTGESDGGYMDMSKDESVDYVPMLDMKGDVKYADIESSNYMAPYDNYVPSAPERTCRATLINESPVL SYMDLVGFSYQVANGMEFLASKNCVHRDLAARNVLICEGKLVKICDFGLARDIMRDSNYISKGSTFLPLKWMAPESIFNSLYTTLSDVWS FGILLWEIFTLGGTPYPELPMNEQFYNAIKRGYRMAQPAHASDEIYEIMQKCWEEKFEIRPPFSQLVLLLERLLGEGYKKKYQQVDEEFL RSDHPAILRSQARLPGFHGLRSPLDTSSVLYTAVQPNEGDNDYIIPLPDPKPEVADEGPLEGSPSLASSTLNEVNTSSTISCDSPLEPQD -------------------------------------------------------------- |
Multiple Sequence Alignment of All Fusion Protein Isoforms |
Top |
Kinase Fusion Protein Functional Features |
 Four levels of functional features of fusion genes Four levels of functional features of fusion genesGo to FGviewer search page for the most frequent breakpoint (https://ccsmweb.uth.edu/FGviewer/chr14:/chr5:) - FGviewer provides the online visualization of the retention search of the protein functional features across DNA, RNA, protein, and pathological levels. - How to search 1. Put your fusion gene symbol. 2. Press the tab key until there will be shown the breakpoint information filled. 4. Go down and press 'Search' tab twice. 4. Go down to have the hyperlink of the search result. 5. Click the hyperlink. 6. See the FGviewer result for your fusion gene. |
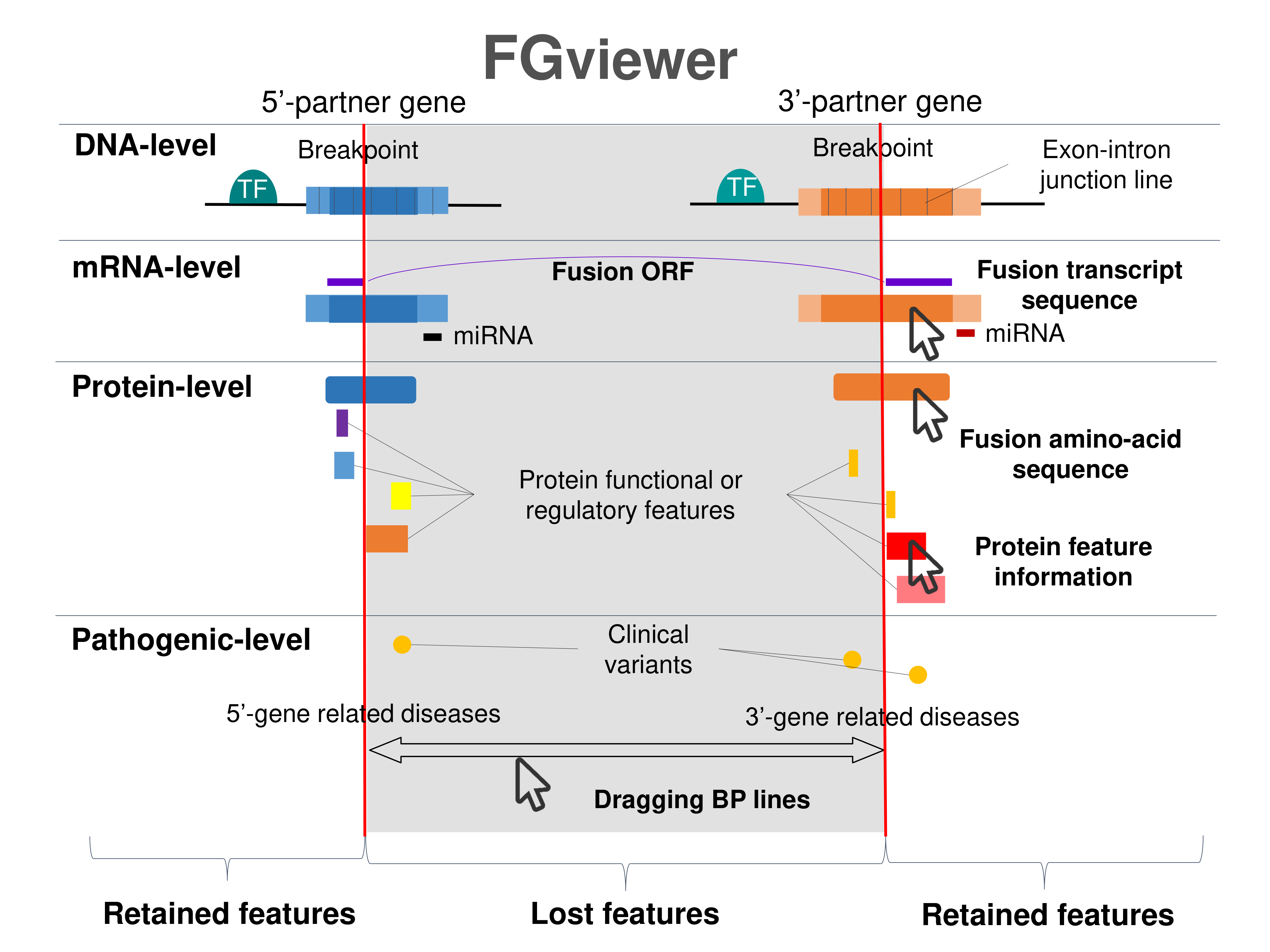 |
 Main function of each fusion partner protein. (from UniProt) Main function of each fusion partner protein. (from UniProt) |
| Hgene | Tgene |
| TRIP11 | PDGFRB |
| FUNCTION: Is a membrane tether required for vesicle tethering to Golgi. Has an essential role in the maintenance of Golgi structure and function (PubMed:25473115, PubMed:30728324). It is required for efficient anterograde and retrograde trafficking in the early secretory pathway, functioning at both the ER-to-Golgi intermediate compartment (ERGIC) and Golgi complex (PubMed:25717001). Binds the ligand binding domain of the thyroid receptor (THRB) in the presence of triiodothyronine and enhances THRB-modulated transcription. {ECO:0000269|PubMed:10189370, ECO:0000269|PubMed:25473115, ECO:0000269|PubMed:25717001, ECO:0000269|PubMed:30728324, ECO:0000269|PubMed:9256431}. | FUNCTION: Tyrosine-protein kinase that acts as a cell-surface receptor for homodimeric PDGFB and PDGFD and for heterodimers formed by PDGFA and PDGFB, and plays an essential role in the regulation of embryonic development, cell proliferation, survival, differentiation, chemotaxis and migration. Plays an essential role in blood vessel development by promoting proliferation, migration and recruitment of pericytes and smooth muscle cells to endothelial cells. Plays a role in the migration of vascular smooth muscle cells and the formation of neointima at vascular injury sites. Required for normal development of the cardiovascular system. Required for normal recruitment of pericytes (mesangial cells) in the kidney glomerulus, and for normal formation of a branched network of capillaries in kidney glomeruli. Promotes rearrangement of the actin cytoskeleton and the formation of membrane ruffles. Binding of its cognate ligands - homodimeric PDGFB, heterodimers formed by PDGFA and PDGFB or homodimeric PDGFD -leads to the activation of several signaling cascades; the response depends on the nature of the bound ligand and is modulated by the formation of heterodimers between PDGFRA and PDGFRB. Phosphorylates PLCG1, PIK3R1, PTPN11, RASA1/GAP, CBL, SHC1 and NCK1. Activation of PLCG1 leads to the production of the cellular signaling molecules diacylglycerol and inositol 1,4,5-trisphosphate, mobilization of cytosolic Ca(2+) and the activation of protein kinase C. Phosphorylation of PIK3R1, the regulatory subunit of phosphatidylinositol 3-kinase, leads to the activation of the AKT1 signaling pathway. Phosphorylation of SHC1, or of the C-terminus of PTPN11, creates a binding site for GRB2, resulting in the activation of HRAS, RAF1 and down-stream MAP kinases, including MAPK1/ERK2 and/or MAPK3/ERK1. Promotes phosphorylation and activation of SRC family kinases. Promotes phosphorylation of PDCD6IP/ALIX and STAM. Receptor signaling is down-regulated by protein phosphatases that dephosphorylate the receptor and its down-stream effectors, and by rapid internalization of the activated receptor. {ECO:0000269|PubMed:11297552, ECO:0000269|PubMed:11331881, ECO:0000269|PubMed:1314164, ECO:0000269|PubMed:1396585, ECO:0000269|PubMed:1653029, ECO:0000269|PubMed:1709159, ECO:0000269|PubMed:1846866, ECO:0000269|PubMed:20494825, ECO:0000269|PubMed:20529858, ECO:0000269|PubMed:21098708, ECO:0000269|PubMed:21679854, ECO:0000269|PubMed:21733313, ECO:0000269|PubMed:2554309, ECO:0000269|PubMed:26599395, ECO:0000269|PubMed:2835772, ECO:0000269|PubMed:2850496, ECO:0000269|PubMed:7685273, ECO:0000269|PubMed:7691811, ECO:0000269|PubMed:7692233, ECO:0000269|PubMed:8195171}. |
 Retention analysis result of each fusion partner protein across 39 protein features of UniProt such as six molecule processing features, 13 region features, four site features, six amino acid modification features, two natural variation features, five experimental info features, and 3 secondary structure features. Here, because of limited space for viewing, we only show the protein feature retention information belong to the 13 regional features. All retention annotation result can be downloaded at * Minus value of BPloci means that the break pointn is located before the CDS. Retention analysis result of each fusion partner protein across 39 protein features of UniProt such as six molecule processing features, 13 region features, four site features, six amino acid modification features, two natural variation features, five experimental info features, and 3 secondary structure features. Here, because of limited space for viewing, we only show the protein feature retention information belong to the 13 regional features. All retention annotation result can be downloaded at * Minus value of BPloci means that the break pointn is located before the CDS. |
 - Retained domain in the 5'-partner of fusion protein (protein functional feature from UniProt). - Retained domain in the 5'-partner of fusion protein (protein functional feature from UniProt). |
| Partner | Hgeneene | Hbp | Tgeneene | Tbp | ENST | BPexon | TotalExon | Protein feature loci | BPloci | TotalLen | Feature | Note |
 - Retained domain in the 3'-partner of fusion protein (protein functional feature from UniProt). - Retained domain in the 3'-partner of fusion protein (protein functional feature from UniProt). |
| Partner | Hgeneene | Hbp | Tgeneene | Tbp | ENST | BPexon | TotalExon | Protein feature loci | BPloci | TotalLen | Feature | Note |
| Tgene | TRIP11 | 92454627 | PDGFRB | 149506177 | ENST00000267622 | 9 | 23 | 600_962 | 526 | 1107 | Domain | Note=Protein kinase;Ontology_term=ECO:0000255;evidence=ECO:0000255|PROSITE-ProRule:PRU00159 |
Top |
Kinase Fusion Protein Structures |
 CIF files of the predicted kinase fusion proteins CIF files of the predicted kinase fusion proteins * Here we show the 3D structure of the fusion proteins using Mol*. AlphaFold produces a per-residue confidence score (pLDDT) between 0 and 100. Model confidence is shown from the pLDDT values per residue. pLDDT corresponds to the model’s prediction of its score on the local Distance Difference Test. It is a measure of local accuracy (from AlphfaFold website). To color code individual residues, we transformed individual PDB files into CIF format. |
| Kinase Fusion protein CIF link (fusion AA seq ID in KinaseFusionDB) | Henst | Tenst | Hgene | Hchr | Hbp | Tgene | Tchr | Tbp | AA seq | Len(AA seq) |
| PDB file >>>535_TRIP11_PDGFRB | ENST00000267622 | ENST00000261799 | TRIP11 | chr14 | 92454627 | PDGFRB | chr5 | 149506177 | MRTQLCVIVSCLAKAGVQGYLVGSPGGAKRFLFSERTGSFSKLAAMSSWLGGLGSGLGQSLGQVGGSLASLTGQISNFTKDMLMEGTEEV EAELPDSRTKEIEAIHAILRSENERLKKLCTDLEEKHEASEIQIKQQSTSYRNQLQQKEVEISHLKARQIALQDQLLKLQSAAQSVPSGA GVPATTASSSFAYGISHHPSAFHDDDMDFGDIISSQQEINRLSNEVSRLESEVGHWRHIAQTSKAQGTDNSDQSEICKLQNIIKELKQNR SQEIDDHQHEMSVLQNAHQQKLTEISRRHREELSDYEERIEELENLLQQGGSGVIETDLSKIYEMQKTIQVLQIEKVESTKKMEQLEDKI KDINKKLSSAENDRDILRREQEQLNVEKRQIMEECENLKLECSKLQPSAVKQSDTMTEKERILAQSASVEEVFRLQQALSDAENEIMRLS SLNQDNSLAEDNLKLKMRIEVLEKEKSLLSQEKEELQMSLLKLNNEYEVIKSTATRDISLDSELHDLRLNLEAKEQELNQSISEKETLIA EIEELDRQNQEATKHMILIKDQLSKQQNEGDSIISKLKQDLNDEKKRVHQLEDDKMDITKELDVQKEKLIQSEVALNDLHLTKQKLEDKV ENLVDQLNKSQESNVSIQKENLELKEHIRQNEEELSRIRNELMQSLNQDSNSNFKDTLLKEREAEVRNLKQNLSELEQLNENLKKVAFDV KMENEKLVLACEDVRHQLEECLAGNNQLSLEKNTIVETLKMEKGEIEAELCWAKKRLLEEANKYEKTIEELSNARNLNTSALQLEHEHLI KLNQKKDMEIAELKKNIEQMDTDHKETKDVLSSSLEEQKQLTQLINKKEIFIEKLKERSSKLQEELDKYSQALRKNEILRQTIEEKDRSL GSMKEENNHLQEELERLREEQSRTAPVADPKTLDSVTELASEVSQLNTIKEHLEEEIKHHQKIIEDQNQSKMQLLQSLQEQKKEMDEFRY QHEQMNATHTQLFLEKDEEIKSLQKTIEQIKTQLHEERQDIQTDNSDIFQETKVQSLNIENGSEKHDLSKAETERLVKGIKERELEIKLL NEKNISLTKQIDQLSKDEVGKLTQIIQQKDLEIQALHARISSTSHTQDVVYLQQQLQAYAMEREKVFAVLNEKTRENSHLKTEYHKMMDI VAAKEAALIKLQDENKKLSTRFESSGQDMFRETIQNLSRIIREKDIEIDALSQKCQTLLAVLQTSSTGNEAGGVNSNQFEELLQERDKLK QQVKKMEEWKQQVMTTVQNMQHESAQLQEELHQLQAQVLVDSDNNSKLQVDYTGLIQSYEQNETKLKNFGQELAQVQHSIGQLCNTKDLL LGKLDIISPQLSSASLLTPQSAECLRASKSEVLSESSELLQQELEELRKSLQEKDATIRTLQENNHRLSDSIAATSELERKEHEQTDSEI KQLKEKQDVLQKLLKEKDLLIKAKSDQLLSSNENFTNKVNENELLRQAVTNLKERILILEMDIGKLKGENEKIVETYRGKETEYQALQET NMKFSMMLREKEFECHSMKEKALAFEQLLKEKEQGKTGELNQLLNAVKSMQEKTVVFQQERDQVMLALKQKQMENTALQNEVQRLRDKEF RSNQELERLRNHLLESEDSYTREALAAEDREAKLRKKVTVLEEKLVSSSNAMENASHQASVQVESLQEQLNVVSKQRDETALQLSVSQEQ VKQYALSLANLQMVLEHFQQEEKAMYSAELEKQKQLIAEWKKNAENLEGKVISLQECLDEANAALDSASRLTEQLDVKEEQIEELKRQTL PFKVVVISAILALVVLTIISLIILIMLWQKKPRYEIRWKVIESVSSDGHEYIYVDPMQLPYDSTWELPRDQLVLGRTLGSGAFGQVVEAT AHGLSHSQATMKVAVKMLKSTARSSEKQALMSELKIMSHLGPHLNVVNLLGACTKGGPIYIITEYCRYGDLVDYLHRNKHTFLQHHSDKR RPPSAELYSNALPVGLPLPSHVSLTGESDGGYMDMSKDESVDYVPMLDMKGDVKYADIESSNYMAPYDNYVPSAPERTCRATLINESPVL SYMDLVGFSYQVANGMEFLASKNCVHRDLAARNVLICEGKLVKICDFGLARDIMRDSNYISKGSTFLPLKWMAPESIFNSLYTTLSDVWS FGILLWEIFTLGGTPYPELPMNEQFYNAIKRGYRMAQPAHASDEIYEIMQKCWEEKFEIRPPFSQLVLLLERLLGEGYKKKYQQVDEEFL RSDHPAILRSQARLPGFHGLRSPLDTSSVLYTAVQPNEGDNDYIIPLPDPKPEVADEGPLEGSPSLASSTLNEVNTSSTISCDSPLEPQD | 2378 |
| 3D view using mol* of 535_TRIP11_PDGFRB | ||||||||||
| PDB file >>>TKFP_918_TRIP11_PDGFRB | ENST00000267622 | ENST00000261799 | TRIP11 | chr14 | 92454627 | PDGFRB | chr5 | 149506177 | MRTQLCVIVSCLAKAGVQGYLVGSPGGAKRFLFSERTGSFSKLAAMSSWLGGLGSGLGQSLGQVGGSLASLTGQISNFTKDMLMEGTEEV EAELPDSRTKEIEAIHAILRSENERLKKLCTDLEEKHEASEIQIKQQSTSYRNQLQQKEVEISHLKARQIALQDQLLKLQSAAQSVPSGA GVPATTASSSFAYGISHHPSAFHDDDMDFGDIISSQQEINRLSNEVSRLESEVGHWRHIAQTSKAQGTDNSDQSEICKLQNIIKELKQNR SQEIDDHQHEMSVLQNAHQQKLTEISRRHREELSDYEERIEELENLLQQGGSGVIETDLSKIYEMQKTIQVLQIEKVESTKKMEQLEDKI KDINKKLSSAENDRDILRREQEQLNVEKRQIMEECENLKLECSKLQPSAVKQSDTMTEKERILAQSASVEEVFRLQQALSDAENEIMRLS SLNQDNSLAEDNLKLKMRIEVLEKEKSLLSQEKEELQMSLLKLNNEYEVIKSTATRDISLDSELHDLRLNLEAKEQELNQSISEKETLIA EIEELDRQNQEATKHMILIKDQLSKQQNEGDSIISKLKQDLNDEKKRVHQLEDDKMDITKELDVQKEKLIQSEVALNDLHLTKQKLEDKV ENLVDQLNKSQESNVSIQKENLELKEHIRQNEEELSRIRNELMQSLNQDSNSNFKDTLLKEREAEVRNLKQNLSELEQLNENLKKVAFDV KMENEKLVLACEDVRHQLEECLAGNNQLSLEKNTIVETLKMEKGEIEAELCWAKKRLLEEANKYEKTIEELSNARNLNTSALQLEHEHLI KLNQKKDMEIAELKKNIEQMDTDHKETKDVLSSSLEEQKQLTQLINKKEIFIEKLKERSSKLQEELDKYSQALRKNEILRQTIEEKDRSL GSMKEENNHLQEELERLREEQSRTAPVADPKTLDSVTELASEVSQLNTIKEHLEEEIKHHQKIIEDQNQSKMQLLQSLQEQKKEMDEFRY QHEQMNATHTQLFLEKDEEIKSLQKTIEQIKTQLHEERQDIQTDNSDIFQETKVQSLNIENGSEKHDLSKAETERLVKGIKERELEIKLL NEKNISLTKQIDQLSKDEVGKLTQIIQQKDLEIQALHARISSTSHTQDVVYLQQQLQAYAMEREKVFAVLNEKTRENSHLKTEYHKMMDI VAAKEAALIKLQDENKKLSTRFESSGQDMFRETIQNLSRIIREKDIEIDALSQKCQTLLAVLQTSSTGNEAGGVNSNQFEELLQERDKLK QQVKKMEEWKQQVMTTVQNMQHESAQLQEELHQLQAQVLVDSDNNSKLQVDYTGLIQSYEQNETKLKNFGQELAQVQHSIGQLCNTKDLL LGKLDIISPQLSSASLLTPQSAECLRASKSEVLSESSELLQQELEELRKSLQEKDATIRTLQENNHRLSDSIAATSELERKEHEQTDSEI KQLKEKQDVLQKLLKEKDLLIKAKSDQLLSSNENFTNKVNENELLRQAVTNLKERILILEMDIGKLKGENEKIVETYRGKETEYQALQET NMKFSMMLREKEFECHSMKEKALAFEQLLKEKEQGKTGELNQLLNAVKSMQEKTVVFQQERDQVMLALKQKQMENTALQNEVQRLRDKEF RSNQELERLRNHLLESEDSYTREALAAEDREAKLRKKVTVLEEKLVSSSNAMENASHQASVQVESLQEQLNVVSKQRDETALQLSVSQEQ VKQYALSLANLQMVLEHFQQEEKAMYSAELEKQKQLIAEWKKNAENLEGKVISLQECLDEANAALDSASRLTEQLDVKEEQIEELKRQTL PFKVVVISAILALVVLTIISLIILIMLWQKKPRYEIRWKVIESVSSDGHEYIYVDPMQLPYDSTWELPRDQLVLGRTLGSGAFGQVVEAT AHGLSHSQATMKVAVKMLKSTARSSEKQALMSELKIMSHLGPHLNVVNLLGACTKGGPIYIITEYCRYGDLVDYLHRNKHTFLQHHSDKR RPPSAELYSNALPVGLPLPSHVSLTGESDGGYMDMSKDESVDYVPMLDMKGDVKYADIESSNYMAPYDNYVPSAPERTCRATLINESPVL SYMDLVGFSYQVANGMEFLASKNCVHRDLAARNVLICEGKLVKICDFGLARDIMRDSNYISKGSTFLPLKWMAPESIFNSLYTTLSDVWS FGILLWEIFTLGGTPYPELPMNEQFYNAIKRGYRMAQPAHASDEIYEIMQKCWEEKFEIRPPFSQLVLLLERLLGEGYKKKYQQVDEEFL RSDHPAILRSQARLPGFHGLRSPLDTSSVLYTAVQPNEGDNDYIIPLPDPKPEVADEGPLEGSPSLASSTLNEVNTSSTISCDSPLEPQD | 2378_TRIP11_PDGFRB |
Top |
Comparison of Fusion Protein Isoforms |
 Superimpose the 3D Structures Among All Fusion Protein Isoforms Superimpose the 3D Structures Among All Fusion Protein Isoforms * Download the pdb file and open it from the molstar online viewer. |
 Comparison of the Secondary Structures of Fusion Protein Isoforms Comparison of the Secondary Structures of Fusion Protein Isoforms |
Top |
Comparison of Fusion Protein Sequences/Structures with Known Sequences/Structures from PDB |
Top |
pLDDT score distribution |
 pLDDT score distribution of the predicted fusion protein structures from AlphaFold2 pLDDT score distribution of the predicted fusion protein structures from AlphaFold2* AlphaFold produces a per-residue confidence score (pLDDT) between 0 and 100. * The blue color at the bottom marks the best active site residues. |
| 535_TRIP11_PDGFRB.png |
 |
| 535_TRIP11_PDGFRB.png |
 |
Top |
Potential Active Site Information |
 The potential binding sites of these fusion proteins were identified using SiteMap, a module of the Schrodinger suite. The potential binding sites of these fusion proteins were identified using SiteMap, a module of the Schrodinger suite. |
| Kinase Fusion AA seq ID in KinaseFusionDB | Site score | Size | Dscore | Volume | Exposure | Enclosure | Contact | Phobic | Philic | Balance | Don/Acc | Residues |
Top |
Ramachandran Plot of Kinase Fusion Protein Structure |
 Ramachandran plot of the torsional angles - phi (φ)and psi (ψ) - of the residues (amino acids) contained in this fusion protein peptide. Ramachandran plot of the torsional angles - phi (φ)and psi (ψ) - of the residues (amino acids) contained in this fusion protein peptide. |
| 535_TRIP11_PDGFRB_ramachandran.png |
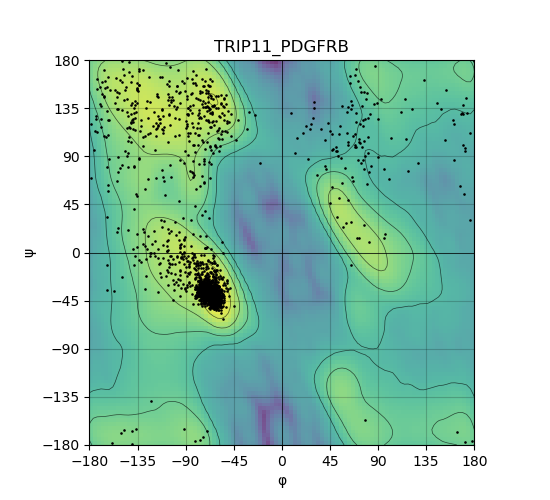 |
Top |
Virtual Screening Results |
 Distribution of the average docking score across all approved kinase inhibitors. Distribution of the average docking score across all approved kinase inhibitors.Distribution of the number of occurrence across all approved kinase inhibitors. |
| 5'-kinase fusion protein case |
| 3'-kinase fusion protein case |
 |
Top |
 Drug information from DrugBank of the top 20 interacting small molecules. Drug information from DrugBank of the top 20 interacting small molecules.* The detailed information of individual kinase inhibitors are available in the download page. |
| Fusion gene name info | Drug | Docking score | Glide g score | Glide energy |
| 535_TRIP11_PDGFRB-DOCK_HTVS_1-001 | Midostaurin | -9.19721 | -9.19721 | -56.2072 |
| 535_TRIP11_PDGFRB-DOCK_HTVS_1-001 | Netarsudil | -8.5274 | -8.5385 | -60.1611 |
| 535_TRIP11_PDGFRB-DOCK_HTVS_1-001 | Netarsudil | -8.5274 | -8.5385 | -60.1611 |
| 535_TRIP11_PDGFRB-DOCK_HTVS_1-001 | Afatinib | -8.30434 | -8.48664 | -55.4876 |
| 535_TRIP11_PDGFRB-DOCK_HTVS_1-001 | Afatinib | -8.30434 | -8.48664 | -55.4876 |
| 535_TRIP11_PDGFRB-DOCK_HTVS_1-001 | Afatinib | -8.30294 | -8.48664 | -55.4876 |
| 535_TRIP11_PDGFRB-DOCK_HTVS_1-001 | Vandetanib | -8.18033 | -8.18033 | -46.2436 |
| 535_TRIP11_PDGFRB-DOCK_HTVS_1-001 | Erdafitinib | -8.17609 | -8.17629 | -49.2838 |
| 535_TRIP11_PDGFRB-DOCK_HTVS_1-001 | Larotrectinib | -7.998289999999999 | -7.998289999999999 | -50.3116 |
| 535_TRIP11_PDGFRB-DOCK_HTVS_1-001 | Neratinib | -7.98525 | -8.17115 | -70.3274 |
| 535_TRIP11_PDGFRB-DOCK_HTVS_1-001 | Avapritinib | -7.861669999999999 | -8.192969999999999 | -50.9237 |
| 535_TRIP11_PDGFRB-DOCK_HTVS_1-001 | Avapritinib | -7.861669999999999 | -8.192969999999999 | -50.9237 |
| 535_TRIP11_PDGFRB-DOCK_HTVS_1-001 | Avapritinib | -7.861669999999999 | -8.192969999999999 | -50.9237 |
| 535_TRIP11_PDGFRB-DOCK_HTVS_1-001 | Avapritinib | -7.861669999999999 | -8.192969999999999 | -50.9237 |
| 535_TRIP11_PDGFRB-DOCK_HTVS_1-001 | Afatinib | -7.81646 | -7.998760000000001 | -55.1729 |
| 535_TRIP11_PDGFRB-DOCK_HTVS_1-001 | Afatinib | -7.81646 | -7.998760000000001 | -55.1729 |
| 535_TRIP11_PDGFRB-DOCK_HTVS_1-001 | Afatinib | -7.815060000000001 | -7.998760000000001 | -55.1729 |
| 535_TRIP11_PDGFRB-DOCK_HTVS_1-001 | Palbociclib | -7.695589999999999 | -8.16509 | -59.5838 |
| 535_TRIP11_PDGFRB-DOCK_HTVS_1-001 | Palbociclib | -7.695589999999999 | -8.16509 | -59.5838 |
| 535_TRIP11_PDGFRB-DOCK_HTVS_1-001 | Palbociclib | -7.695589999999999 | -8.16509 | -59.5838 |
Top |
Kinase-Substrate Information of TRIP11_PDGFRB |
 Phosphorylation target of the kinase Phosphorylation target of the kinase(phosphosite, 03-17-2024) |
| Kinase | Kinase UniProt Acc | Kinase species | Substrate | Substrate UniProt Acc | Substrate phosphorylated residues | Substrate phosphorylated sites (+/-7AA) | Domain |
| PDGFRB | P09619 | human | PDGFRB | P09619 | Y1021 | PNEGDNDyIIPLPDP | |
| PDGFRB | P09619 | human | PDGFRA | P16234 | Y754 | ERKEVsKysDIQRsL | PK_Tyr_Ser-Thr |
| PDGFRB | P09619 | human | PDGFRB | P09619 | Y1009 | LDTSSVLyTAVQPNE | |
| PDGFRB | P09619 | human | PTK2 | Q05397 | Y5 | ___MAAAyLDPNLNH | |
| PDGFRB | P09619 | human | ABL2 | P42684 | Y272 | KCNKPTVyGVsPIHD | |
| PDGFRB | P09619 | human | PDGFRB | P09619 | Y579 | VSSDGHEyIyVDPMQ | |
| PDGFRB | P09619 | human | PDGFRB | P09619 | Y562 | LWQKKPRyEIRWKVI | |
| PDGFRB | P09619 | human | THOC5 | Q13769 | Y225 | EIEVKKEyLSSLQPR | FmiP_Thoc5 |
| PDGFRB | P09619 | human | ABL2 | P42684 | Y139 | EKLRVLGyNQNGEWS | SH3_1 |
| PDGFRB | P09619 | human | PLCG1 | P19174 | Y783 | EGRNPGFyVEANPMP | |
| PDGFRB | P09619 | human | PDGFRB | P09619 | Y740 | TGESDGGyMDMSKDE | PK_Tyr_Ser-Thr |
| PDGFRB | P09619 | human | ABL2 | P42684 | Y439 | RLMtGDtytAHAGAk | PK_Tyr_Ser-Thr |
| PDGFRB | P09619 | human | FYN | P06241 | Y28 | sLNQssGyRyGTDPT | |
| PDGFRB | P09619 | human | ETV6 | P41212 | Y17 | IkQERIsytPPEsPV | |
| PDGFRB | P09619 | human | PTK2 | Q05397 | Y194 | ALEKKSNyEVLEkDV | FERM_M |
| PDGFRB | P09619 | human | PDGFRB | P09619 | Y763 | DMKGDVKyADIESSN | PK_Tyr_Ser-Thr |
| PDGFRB | P09619 | human | TNK2 | Q07912 | Y635 | PLPPPPAyDDVAQDE | |
| PDGFRB | P09619 | human | PDGFRB | P09619 | Y581 | SDGHEyIyVDPMQLP | |
| PDGFRB | P09619 | human | ABL2 | P42684 | Y116 | PNLFVALyDFVAsGD | SH3_1 |
| PDGFRB | P09619 | human | ABL2 | P42684 | Y303 | GGQyGEVyVGVWKKy | PK_Tyr_Ser-Thr |
| PDGFRB | P09619 | human | MUC1 | P15941 | Y1218 | tyHtHGRyVPPsstD | |
| PDGFRB | P09619 | human | ABL2 | P42684 | Y310 | yVGVWKKysLtVAVK | PK_Tyr_Ser-Thr |
| PDGFRB | P09619 | human | PDGFRB | P09619 | Y857 | DIMRDSNyISKGSTF | PK_Tyr_Ser-Thr |
| PDGFRB | P09619 | human | PDGFRB | P09619 | Y775 | SSNyMAPyDNyVPSA | PK_Tyr_Ser-Thr |
| PDGFRB | P09619 | human | MUC1 | P15941 | Y1203 | IFPARDtyHPMsEyP | |
| PDGFRB | P09619 | human | PDGFRB | P09619 | Y771 | ADIESSNyMAPyDNy | PK_Tyr_Ser-Thr |
| PDGFRB | P09619 | human | ABL2 | P42684 | Y161 | QGWVPsNyItPVNSL | |
| PDGFRB | P09619 | human | ETV6 | P41212 | Y27 | PEsPVPsyAsstPLH | |
| PDGFRB | P09619 | human | SRC | P12931 | Y419 | RLIEDNEytARQGAk | PK_Tyr_Ser-Thr |
| PDGFRB | P09619 | human | PDGFRB | P09619 | Y751 | SKDESVDyVPMLDMK | PK_Tyr_Ser-Thr |
| PDGFRB | P09619 | human | ABL2 | P42684 | Y299 | HKLGGGQyGEVyVGV | PK_Tyr_Ser-Thr |
| PDGFRB | P09619 | human | PDGFRB | P09619 | Y778 | yMAPyDNyVPSAPER | PK_Tyr_Ser-Thr |
 Biological Network Integration of This Kinase and Substrates Biological Network Integration of This Kinase and Substrates (GeneMANIA website) |
 Enriched GO biological processes of the phosphorylation target genes of the kinase Enriched GO biological processes of the phosphorylation target genes of the kinase |
| Kinase | GOID | GO term | P.adjust |
| PDGFRB | ID | Description | 0.00e+00 |
| PDGFRB | GO:0018108 | peptidyl-tyrosine phosphorylation | 1.87e-08 |
| PDGFRB | GO:0018212 | peptidyl-tyrosine modification | 1.87e-08 |
| PDGFRB | GO:0038093 | Fc receptor signaling pathway | 6.42e-06 |
| PDGFRB | GO:0036120 | cellular response to platelet-derived growth factor stimulus | 4.63e-05 |
| PDGFRB | GO:0036119 | response to platelet-derived growth factor | 4.63e-05 |
| PDGFRB | GO:0002433 | immune response-regulating cell surface receptor signaling pathway involved in phagocytosis | 4.63e-05 |
| PDGFRB | GO:0038096 | Fc-gamma receptor signaling pathway involved in phagocytosis | 4.63e-05 |
| PDGFRB | GO:0002431 | Fc receptor mediated stimulatory signaling pathway | 6.79e-05 |
| PDGFRB | GO:0002862 | negative regulation of inflammatory response to antigenic stimulus | 6.79e-05 |
| PDGFRB | GO:0038094 | Fc-gamma receptor signaling pathway | 6.79e-05 |
| PDGFRB | GO:0010863 | positive regulation of phospholipase C activity | 8.77e-05 |
| PDGFRB | GO:1900274 | regulation of phospholipase C activity | 9.45e-05 |
| PDGFRB | GO:0051897 | positive regulation of phosphatidylinositol 3-kinase/protein kinase B signal transduction | 1.34e-04 |
| PDGFRB | GO:0002861 | regulation of inflammatory response to antigenic stimulus | 1.52e-04 |
| PDGFRB | GO:0010518 | positive regulation of phospholipase activity | 1.52e-04 |
| PDGFRB | GO:0048013 | ephrin receptor signaling pathway | 1.61e-04 |
| PDGFRB | GO:0046777 | protein autophosphorylation | 1.87e-04 |
| PDGFRB | GO:0048008 | platelet-derived growth factor receptor signaling pathway | 2.11e-04 |
| PDGFRB | GO:0010517 | regulation of phospholipase activity | 2.11e-04 |
| PDGFRB | GO:0060193 | positive regulation of lipase activity | 2.11e-04 |
| PDGFRB | GO:0048010 | vascular endothelial growth factor receptor signaling pathway | 2.11e-04 |
| PDGFRB | GO:0051896 | regulation of phosphatidylinositol 3-kinase/protein kinase B signal transduction | 3.46e-04 |
| PDGFRB | GO:0060191 | regulation of lipase activity | 4.05e-04 |
| PDGFRB | GO:0002437 | inflammatory response to antigenic stimulus | 4.19e-04 |
| PDGFRB | GO:0043491 | phosphatidylinositol 3-kinase/protein kinase B signal transduction | 5.64e-04 |
| PDGFRB | GO:0002429 | immune response-activating cell surface receptor signaling pathway | 7.80e-04 |
| PDGFRB | GO:0035791 | platelet-derived growth factor receptor-beta signaling pathway | 8.61e-04 |
| PDGFRB | GO:0007173 | epidermal growth factor receptor signaling pathway | 8.61e-04 |
| PDGFRB | GO:0033674 | positive regulation of kinase activity | 8.77e-04 |
| PDGFRB | GO:0002768 | immune response-regulating cell surface receptor signaling pathway | 9.40e-04 |
| PDGFRB | GO:2001243 | negative regulation of intrinsic apoptotic signaling pathway | 9.40e-04 |
| PDGFRB | GO:0072224 | metanephric glomerulus development | 1.11e-03 |
| PDGFRB | GO:0038127 | ERBB signaling pathway | 1.14e-03 |
| PDGFRB | GO:2000811 | negative regulation of anoikis | 1.18e-03 |
| PDGFRB | GO:0055003 | cardiac myofibril assembly | 1.24e-03 |
| PDGFRB | GO:0061298 | retina vasculature development in camera-type eye | 1.24e-03 |
| PDGFRB | GO:0050900 | leukocyte migration | 1.29e-03 |
| PDGFRB | GO:0043552 | positive regulation of phosphatidylinositol 3-kinase activity | 1.45e-03 |
| PDGFRB | GO:0051347 | positive regulation of transferase activity | 1.46e-03 |
| PDGFRB | GO:0050921 | positive regulation of chemotaxis | 1.49e-03 |
| PDGFRB | GO:0002223 | stimulatory C-type lectin receptor signaling pathway | 1.54e-03 |
| PDGFRB | GO:1990840 | response to lectin | 1.54e-03 |
| PDGFRB | GO:1990858 | cellular response to lectin | 1.54e-03 |
| PDGFRB | GO:0034614 | cellular response to reactive oxygen species | 1.60e-03 |
| PDGFRB | GO:2000209 | regulation of anoikis | 1.60e-03 |
| PDGFRB | GO:0090218 | positive regulation of lipid kinase activity | 1.85e-03 |
| PDGFRB | GO:0050731 | positive regulation of peptidyl-tyrosine phosphorylation | 1.97e-03 |
| PDGFRB | GO:0002757 | immune response-activating signaling pathway | 1.99e-03 |
| PDGFRB | GO:0032102 | negative regulation of response to external stimulus | 2.07e-03 |
Top |
Related Drugs to TRIP11_PDGFRB |
 Drugs used for this fusion-positive patient. Drugs used for this fusion-positive patient. (Manual curation of PubMed, 04-30-2022 + MyCancerGenome) |
| Hgene | Tgene | Drug | Source | PMID |
 Distribution of the number of studies mentioning TRIP11-PDGFRB and kinase inhibitors the PubMed Abstract (04-01-2024) Distribution of the number of studies mentioning TRIP11-PDGFRB and kinase inhibitors the PubMed Abstract (04-01-2024) |
| Fusion gene - drug pair 1 | Fusion gene - drug pair 2 | PMID | Publication date | DOI | Study title |
Top |
Related Diseases to TRIP11_PDGFRB |
 Diseases that have this fusion gene. Diseases that have this fusion gene. (Manual curation of PubMed, 04-30-2022 + MyCancerGenome) |
| Hgene | Tgene | Disease | Source | PMID |
 Related diseases from the literature mentioned this fusion gene and drug. Related diseases from the literature mentioned this fusion gene and drug. (PubMed, 04-01-2024) |
| MeSH ID | MeSH term |
 Diseases associated with fusion partners. Diseases associated with fusion partners. (DisGeNet 4.0) |
| Partner | Gene | Disease ID | Disease name | # pubmeds | Source |
| Tgene | PDGFRB | C3554321 | BASAL GANGLIA CALCIFICATION, IDIOPATHIC, 4 | 6 | CTD_human;GENOMICS_ENGLAND;UNIPROT |
| Tgene | PDGFRB | C0393590 | Fahr's syndrome (disorder) | 3 | GENOMICS_ENGLAND;ORPHANET |
| Tgene | PDGFRB | C4225270 | Kosaki overgrowth syndrome | 3 | CTD_human;GENOMICS_ENGLAND;ORPHANET;UNIPROT |
| Tgene | PDGFRB | C4551572 | MYOFIBROMATOSIS, INFANTILE, 1 | 3 | GENOMICS_ENGLAND;UNIPROT |
| Tgene | PDGFRB | C0013421 | Dystonia | 2 | GENOMICS_ENGLAND |
| Tgene | PDGFRB | C0023480 | Leukemia, Myelomonocytic, Chronic | 2 | ORPHANET |
| Tgene | PDGFRB | C0023893 | Liver Cirrhosis, Experimental | 2 | CTD_human |
| Tgene | PDGFRB | C0036341 | Schizophrenia | 2 | PSYGENET |
| Tgene | PDGFRB | C0432284 | Infantile myofibromatosis | 2 | CTD_human;GENOMICS_ENGLAND;ORPHANET |
Top |
Clinical Trials of the Found Drugs/Small Molecules |
 Statistics of the Clinical Trials of the Found Kinase Inibitors from clinicaltrials.gov (06-17-2024) Statistics of the Clinical Trials of the Found Kinase Inibitors from clinicaltrials.gov (06-17-2024) |
 Clinical Trials from clinicaltrials.gov (06-17-2024) Clinical Trials from clinicaltrials.gov (06-17-2024) |
| Fusion Gene | Kinase Inhibitor | NCT ID | Study Status | Phases | Disease | # Enrolment | Date |

