| UTHEALTH HOME ABOUT SBMI A-Z WEBMAIL INSIDE THE UNIVERSITY |

|
|||||||
|
Kinase Fusion Gene:CAMK2D_CAMK2A |
Kinase Fusion Protein Summary |
 Kinase Fusion gene summary Kinase Fusion gene summary |
| Kinase Fusion partner gene information | Kinase Fusion gene name: CAMK2D_CAMK2A | KinaseFusionDB ID: KFG872 | FusionGDB2.0 ID: KFG872 | Hgene | Tgene | Gene symbol | CAMK2D | CAMK2A | Gene ID | 817 | 815 | |
| Gene name | calcium/calmodulin dependent protein kinase II delta | calcium/calmodulin dependent protein kinase II alpha | ||||||||||
| Synonyms | CAMKD | CAMKA|CaMKIINalpha|CaMKIIalpha|MRD53|MRT63 | ||||||||||
| Cytomap | 4q26 | 5q32 | ||||||||||
| Type of gene | protein-coding | protein-coding | ||||||||||
| Description | calcium/calmodulin-dependent protein kinase type II subunit deltaCaM kinase II delta subunitCaM-kinase II delta chainCaMK-II delta subunitcalcium/calmodulin-dependent protein kinase (CaM kinase) II deltacalcium/calmodulin-dependent protein kinase typ | calcium/calmodulin-dependent protein kinase type II subunit alphaCaM kinase II alpha subunitCaM-kinase II alpha chainCaMK-II alpha subunitcaM kinase II subunit alphacaMK-II subunit alphacalcium/calmodulin-dependent protein kinase (CaM kinase) II alp | ||||||||||
| Modification date | 20240407 | 20240407 | ||||||||||
| UniProtAcc | Q13557 | Q9UQM7 | ||||||||||
| Ensembl transtripts involved in fusion gene | ENST ids | ENST00000296402, ENST00000342666, ENST00000379773, ENST00000394522, ENST00000394524, ENST00000394526, ENST00000418639, ENST00000429180, ENST00000454265, ENST00000508738, ENST00000514328, ENST00000515496, ENST00000505990, ENST00000509907, ENST00000511664, | ENST00000351010, ENST00000398376, ENST00000348628, | |||||||||
| Context (manual curation of fusion genes in KinaseFusionDB) | PubMed: CAMK2D [Title/Abstract] AND CAMK2A [Title/Abstract] AND fusion [Title/Abstract] | |||||||||||
| Most frequent breakpoint (based on all fusion genes of FusionGDB 2.0) | CAMK2D(114469812)-CAMK2A(149636208), # samples:1 | |||||||||||
 Gene ontology of each fusion partner gene with evidence of Inferred from Direct Assay (IDA) from Entrez Gene ontology of each fusion partner gene with evidence of Inferred from Direct Assay (IDA) from Entrez |
| Partner | Gene | GO ID | GO term | PubMed ID |
| Hgene | CAMK2D | GO:0003254 | regulation of membrane depolarization | 22514276 |
| Hgene | CAMK2D | GO:0006468 | protein phosphorylation | 17179159|23283722 |
| Hgene | CAMK2D | GO:0018105 | peptidyl-serine phosphorylation | 22514276|23283722 |
| Hgene | CAMK2D | GO:0018107 | peptidyl-threonine phosphorylation | 22514276|23283722 |
| Hgene | CAMK2D | GO:0046777 | protein autophosphorylation | 22514276 |
| Hgene | CAMK2D | GO:1901897 | regulation of relaxation of cardiac muscle | 23283722 |
| Hgene | CAMK2D | GO:1902306 | negative regulation of sodium ion transmembrane transport | 22514276 |
| Hgene | CAMK2D | GO:2000650 | negative regulation of sodium ion transmembrane transporter activity | 22514276 |
| Tgene | CAMK2A | GO:0006468 | protein phosphorylation | 17052756 |
| Tgene | CAMK2A | GO:0035458 | cellular response to interferon-beta | 35568036 |
| Tgene | CAMK2A | GO:0038166 | angiotensin-activated signaling pathway | 20584908 |
| Tgene | CAMK2A | GO:0046427 | positive regulation of receptor signaling pathway via JAK-STAT | 11972023|35568036 |
| Tgene | CAMK2A | GO:0071346 | cellular response to type II interferon | 11972023 |
 Kinase Fusion gene breakpoints across CAMK2D (5'-gene) Kinase Fusion gene breakpoints across CAMK2D (5'-gene)* Click on the image to open the UCSC genome browser with custom track showing this image in a new window. |
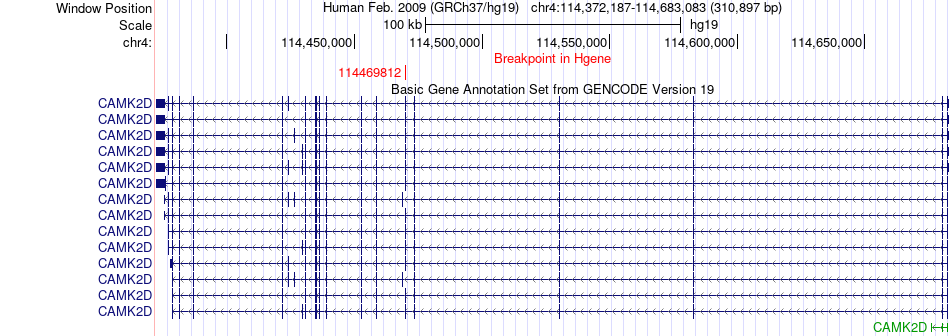 |
 Kinase Fusion gene breakpoints across CAMK2A (3'-gene) Kinase Fusion gene breakpoints across CAMK2A (3'-gene)* Click on the image to open the UCSC genome browser with custom track showing this image in a new window. |
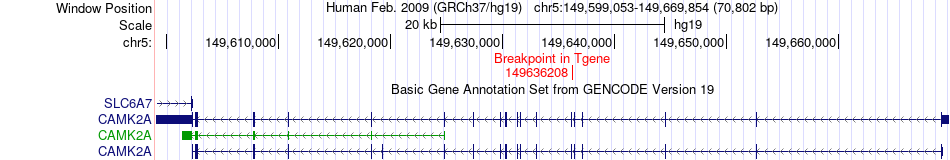 |
Top |
Kinase Fusion Gene Sample Information |
 Kinase Fusion gene information. Kinase Fusion gene information. |
 Kinase Fusion gene information from four resources (ChiTars 5.0, ChimerDB 4.0, COSMIC, and CCLE) Kinase Fusion gene information from four resources (ChiTars 5.0, ChimerDB 4.0, COSMIC, and CCLE)* All genome coordinats were lifted-over on hg19. * Click on the break point to see the gene structure around the break point region using the UCSC Genome Browser. |
| Source | Sample | Hgene | Hchr | Hbp | Tgene | Tchr | Tbp |
| ChimerDB4 | 2397N | CAMK2D | chr4 | 114469812 | CAMK2A | chr5 | 149636208 |
Top |
Kinase Fusion ORF Analysis |
 Kinase Fusion information from ORFfinder translation from full-length transcript sequence from KinaseFusionDB. Kinase Fusion information from ORFfinder translation from full-length transcript sequence from KinaseFusionDB. |
| Henst | Tenst | Hgene | Hchr | Hbp | Tgene | Tchr | Tbp | Seq length (transcript) | Seq length (amino acids) |
Top |
Kinase Fusion Amino Acid Sequences |
 For individual full-length fusion transcript sequence from KinaseFusionDB, we ran ORFfinder and chose the longest ORF among the all predicted ones. For individual full-length fusion transcript sequence from KinaseFusionDB, we ran ORFfinder and chose the longest ORF among the all predicted ones. |
| >Henst_Tenst_Hgene_Hchr_Hbp_Tgene_Tchr_Tbp_length(fusion AA)_AAseq |
Multiple Sequence Alignment of All Fusion Protein Isoforms |
Top |
Kinase Fusion Protein Functional Features |
 Four levels of functional features of fusion genes Four levels of functional features of fusion genesGo to FGviewer search page for the most frequent breakpoint (https://ccsmweb.uth.edu/FGviewer/chr4:114469812/chr5:149636208) - FGviewer provides the online visualization of the retention search of the protein functional features across DNA, RNA, protein, and pathological levels. - How to search 1. Put your fusion gene symbol. 2. Press the tab key until there will be shown the breakpoint information filled. 4. Go down and press 'Search' tab twice. 4. Go down to have the hyperlink of the search result. 5. Click the hyperlink. 6. See the FGviewer result for your fusion gene. |
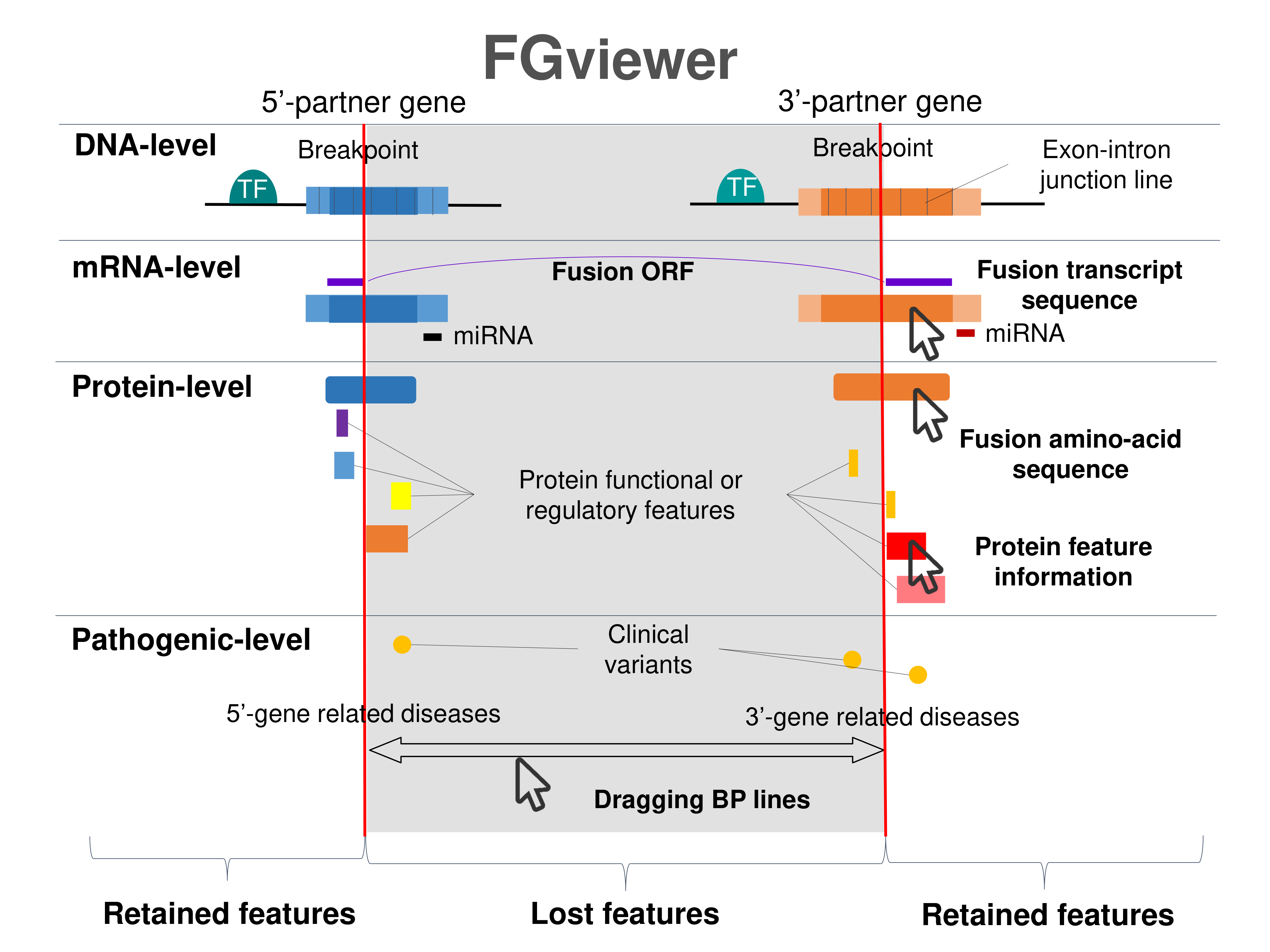 |
 Main function of each fusion partner protein. (from UniProt) Main function of each fusion partner protein. (from UniProt) |
| Hgene | Tgene |
| CAMK2D | CAMK2A |
| FUNCTION: Calcium/calmodulin-dependent protein kinase involved in the regulation of Ca(2+) homeostatis and excitation-contraction coupling (ECC) in heart by targeting ion channels, transporters and accessory proteins involved in Ca(2+) influx into the myocyte, Ca(2+) release from the sarcoplasmic reticulum (SR), SR Ca(2+) uptake and Na(+) and K(+) channel transport. Targets also transcription factors and signaling molecules to regulate heart function. In its activated form, is involved in the pathogenesis of dilated cardiomyopathy and heart failure. Contributes to cardiac decompensation and heart failure by regulating SR Ca(2+) release via direct phosphorylation of RYR2 Ca(2+) channel on 'Ser-2808'. In the nucleus, phosphorylates the MEF2 repressor HDAC4, promoting its nuclear export and binding to 14-3-3 protein, and expression of MEF2 and genes involved in the hypertrophic program (PubMed:17179159). Is essential for left ventricular remodeling responses to myocardial infarction. In pathological myocardial remodeling acts downstream of the beta adrenergic receptor signaling cascade to regulate key proteins involved in ECC. Regulates Ca(2+) influx to myocytes by binding and phosphorylating the L-type Ca(2+) channel subunit beta-2 CACNB2. In addition to Ca(2+) channels, can target and regulate the cardiac sarcolemmal Na(+) channel Nav1.5/SCN5A and the K+ channel Kv4.3/KCND3, which contribute to arrhythmogenesis in heart failure. Phosphorylates phospholamban (PLN/PLB), an endogenous inhibitor of SERCA2A/ATP2A2, contributing to the enhancement of SR Ca(2+) uptake that may be important in frequency-dependent acceleration of relaxation (FDAR) and maintenance of contractile function during acidosis (PubMed:16690701). May participate in the modulation of skeletal muscle function in response to exercise, by regulating SR Ca(2+) transport through phosphorylation of PLN/PLB and triadin, a ryanodine receptor-coupling factor. In response to interferon-gamma (IFN-gamma) stimulation, catalyzes phosphorylation of STAT1, stimulating the JAK-STAT signaling pathway (By similarity). {ECO:0000250|UniProtKB:Q6PHZ2, ECO:0000269|PubMed:16690701, ECO:0000269|PubMed:17179159}. | FUNCTION: Calcium/calmodulin-dependent protein kinase that functions autonomously after Ca(2+)/calmodulin-binding and autophosphorylation, and is involved in various processes, such as synaptic plasticity, neurotransmitter release and long-term potentiation (PubMed:14722083). Member of the NMDAR signaling complex in excitatory synapses, it regulates NMDAR-dependent potentiation of the AMPAR and therefore excitatory synaptic transmission (By similarity). Regulates dendritic spine development (PubMed:28130356). Also regulates the migration of developing neurons (PubMed:29100089). Phosphorylates the transcription factor FOXO3 to activate its transcriptional activity (PubMed:23805378). Phosphorylates the transcription factor ETS1 in response to calcium signaling, thereby decreasing ETS1 affinity for DNA (By similarity). In response to interferon-gamma (IFN-gamma) stimulation, catalyzes phosphorylation of STAT1, stimulating the JAK-STAT signaling pathway (PubMed:11972023). In response to interferon-beta (IFN-beta) stimulation, stimulates the JAK-STAT signaling pathway (PubMed:35568036). Acts as a negative regulator of 2-arachidonoylglycerol (2-AG)-mediated synaptic signaling via modulation of DAGLA activity (By similarity). {ECO:0000250|UniProtKB:P11275, ECO:0000250|UniProtKB:P11798, ECO:0000269|PubMed:11972023, ECO:0000269|PubMed:23805378, ECO:0000269|PubMed:28130356, ECO:0000269|PubMed:29100089}. |
 Retention analysis result of each fusion partner protein across 39 protein features of UniProt such as six molecule processing features, 13 region features, four site features, six amino acid modification features, two natural variation features, five experimental info features, and 3 secondary structure features. Here, because of limited space for viewing, we only show the protein feature retention information belong to the 13 regional features. All retention annotation result can be downloaded at * Minus value of BPloci means that the break pointn is located before the CDS. Retention analysis result of each fusion partner protein across 39 protein features of UniProt such as six molecule processing features, 13 region features, four site features, six amino acid modification features, two natural variation features, five experimental info features, and 3 secondary structure features. Here, because of limited space for viewing, we only show the protein feature retention information belong to the 13 regional features. All retention annotation result can be downloaded at * Minus value of BPloci means that the break pointn is located before the CDS. |
 - Retained domain in the 5'-partner of fusion protein (protein functional feature from UniProt). - Retained domain in the 5'-partner of fusion protein (protein functional feature from UniProt). |
| Partner | Hgeneene | Hbp | Tgeneene | Tbp | ENST | BPexon | TotalExon | Protein feature loci | BPloci | TotalLen | Feature | Note |
 - Retained domain in the 3'-partner of fusion protein (protein functional feature from UniProt). - Retained domain in the 3'-partner of fusion protein (protein functional feature from UniProt). |
| Partner | Hgeneene | Hbp | Tgeneene | Tbp | ENST | BPexon | TotalExon | Protein feature loci | BPloci | TotalLen | Feature | Note |
Top |
Kinase-Substrate Information of CAMK2D_CAMK2A |
 Phosphorylation target of the kinase Phosphorylation target of the kinase(phosphosite, 03-17-2024) |
| Kinase | Kinase UniProt Acc | Kinase species | Substrate | Substrate UniProt Acc | Substrate phosphorylated residues | Substrate phosphorylated sites (+/-7AA) | Domain |
| CAMK2A | Q9UQM7 | human | AKAP5 | P24588 | S92 | LVtRRKRsESsKQQK | WSK |
| CAMK2A | Q9UQM7 | human | SCN5A | Q14524 | T455 | LTIRGVDtVsRssLE | |
| CAMK2A | Q9UQM7 | human | HRH1 | P35367 | T142 | YLKYRtKtRASATIL | 7tm_1 |
| CAMK2A | Q9UQM7 | human | SCN5A | Q14524 | S484 | KRRKRMssGtEECGE | |
| CAMK2A | Q9UQM7 | human | CREB3L3 | Q68CJ9 | S395 | ADTTREEsPGSPGAD | |
| CAMK2A | Q9UQM7 | human | ETS2 | P15036 | S310 | LDVQRVPsFEsFEDD | Ets1_N_flank |
| CAMK2A | Q9UQM7 | human | ITPKA | P23677 | T311 | EHAQRAVtKPRYMQW | IPK |
| CAMK2A | Q9UQM7 | human | SCN5A | Q14524 | S457 | IRGVDtVsRssLEMs | |
| CAMK2A | Q9UQM7 | human | NCOR2 | Q9Y618 | S2426 | AsGDRPPsVSSVHsE | |
| CAMK2A | Q9UQM7 | human | KCNJ11 | Q14654 | T180 | QAHRRAEtLIFSKHA | |
| CAMK2A | Q9UQM7 | human | NOX5 | Q96PH1-4 | S498 | RSVtMRKsQRSsKGS | FAD_binding_8 |
| CAMK2A | Q9UQM7 | human | SCN8A | Q9UQD0 | T642 | RSVKRNstVDCNGVV | Na_trans_cytopl |
| CAMK2A | Q9UQM7 | human | ITGB1 | P05556 | T788 | PIyksAVttVVNPky | Integrin_b_cyt |
| CAMK2A | Q9UQM7 | human | TH | P07101-4 | S35 | AIMVRGQsPRFIGRR | TOH_N |
| CAMK2A | Q9UQM7 | human | SCN5A | Q14524 | S12 | LLPRGTssFRRFtRE | |
| CAMK2A | Q9UQM7 | human | ETS2 | P15036 | S313 | QRVPsFEsFEDDCsQ | Ets1_N_flank |
| CAMK2A | Q9UQM7 | human | SCN5A | Q14524 | S61 | PQLDLQAsKKLPDLY | |
| CAMK2A | Q9UQM7 | human | TCAP | O15273 | S157 | GALRRsLsRSMsQEA | Telethonin |
| CAMK2A | Q9UQM7 | human | SLN | O00631 | T5 | ___MGINtRELFLNF | Sarcolipin |
| CAMK2A | Q9UQM7 | human | CAMK2A | Q9UQM7 | T286 | sCMHRQEtVDCLKKF | |
| CAMK2A | Q9UQM7 | human | SCN5A | Q14524 | S464 | sRssLEMsPLAPVNs | |
| CAMK2A | Q9UQM7 | human | CHAT | P28329-3 | T456 | VDNIRSAtPEALAFV | Carn_acyltransf |
| CAMK2A | Q9UQM7 | human | CAMK2A | Q9UQM7 | T305 | kLkGAILttMLAtRN | |
| CAMK2A | Q9UQM7 | human | ETS1 | P14921 | S285 | QRVPsyDsFDsEDyP | Ets1_N_flank |
| CAMK2A | Q9UQM7 | human | SCN5A | Q14524 | S571 | PWPLRRtsAQGQPsP | Na_trans_cytopl |
| CAMK2A | Q9UQM7 | human | SPR | P35270 | S213 | QQLARETsVDPDMRK | adh_short |
| CAMK2A | Q9UQM7 | human | SCN8A | Q9UQD0 | S541 | NRIGRKFsIMNQsLL | |
| CAMK2A | Q9UQM7 | human | CREB3L3 | Q68CJ9 | S360 | FAPVRVFsRTLHNDA | |
| CAMK2A | Q9UQM7 | human | SLC1A3 | P43003 | T26 | QQGVRKRtLLAKkkV | |
| CAMK2A | Q9UQM7 | human | PTTG1 | O95997 | S31 | LkLGsGPsIkALDGR | Securin |
| CAMK2A | Q9UQM7 | human | SMAD2 | Q15796 | S260 | TLsPVNHsLDLQPVT | |
| CAMK2A | Q9UQM7 | human | TH | P07101-4 | S19 | KGFRRAVsELDAKQA | TOH_N |
| CAMK2A | Q9UQM7 | human | CDKN1B | P46527 | S10 | NVRVSNGsPsLErMD | |
| CAMK2A | Q9UQM7 | human | SRF | P11831 | S103 | RGLKRsLsEMEIGMV | |
| CAMK2A | Q9UQM7 | human | CYLD | Q9NQC7 | S362 | FyTLNGSsVDSQPQs | CYLD_phos_site |
| CAMK2A | Q9UQM7 | human | AKAP5 | P24588 | T87 | AsLKRLVtRRKRsES | WSK |
| CAMK2A | Q9UQM7 | human | RYR2 | Q92736 | S2814 | IsQTSQVsVDAAHGY | |
| CAMK2A | Q9UQM7 | human | SCN5A | Q14524 | S528 | KPRssrGsIFtFRRR | Na_trans_cytopl |
| CAMK2A | Q9UQM7 | human | HDAC5 | Q9UQL6 | S498 | RPLSRtQsSPLPQsP | |
| CAMK2A | Q9UQM7 | human | VIM | P08670 | S83 | GVRLLQDsVdFsLAd | Filament_head |
| CAMK2A | Q9UQM7 | human | ITGB1BP1 | O14713 | T38 | GGLsRsstVAsLDTD | ICAP-1_inte_bdg |
| CAMK2A | Q9UQM7 | human | SCN5A | Q14524 | S497 | GEDRLPKsDsEDGPR | |
| CAMK2A | Q9UQM7 | human | NOX5 | Q96PH1-4 | S475 | WTNRLYEsFKASDPL | FAD_binding_8 |
| CAMK2A | Q9UQM7 | human | SLC6A4 | P31645 | S13 | LNSQKQLsACEDGED | |
| CAMK2A | Q9UQM7 | human | TH | P07101 | S71 | RFIGRRQsLIEDARK | TOH_N |
| CAMK2A | Q9UQM7 | human | SMAD2 | Q15796 | S110 | SFSEQTRsLDGRLQV | MH1 |
| CAMK2A | Q9UQM7 | human | NR1I2 | O75469 | T290 | LCQLRFNtVFNAETG | Hormone_recep |
| CAMK2A | Q9UQM7 | human | FLNA | P21333 | S2523 | VTGPRLVsNHsLHET | |
| CAMK2A | Q9UQM7 | human | BECN1 | Q14457 | S96 | MstEsANsFTLIGEA | |
| CAMK2A | Q9UQM7 | human | PPP1R14A | Q96A00 | S130 | GLRQPsPsHDGsLsP | PP1_inhibitor |
| CAMK2A | Q9UQM7 | human | KCNQ2 | O43526-4 | S438 | QTVRRsPsADQSLED | KCNQ_channel |
| CAMK2A | Q9UQM7 | human | ETS1 | P14921 | S282 | NsLQRVPsyDsFDsE | Ets1_N_flank |
| CAMK2A | Q9UQM7 | human | SCN5A | Q14524 | S1925 | QRsLKHAsFLFRQQA | |
| CAMK2A | Q9UQM7 | human | SCN5A | Q14524 | S510 | PRAMNHLsLTrGLsR | Na_trans_cytopl |
| CAMK2A | Q9UQM7 | human | GFPT1 | Q06210 | S261 | CNLsRVDsttCLFPV | |
| CAMK2A | Q9UQM7 | human | SCN5A | Q14524 | S11 | FLLPRGTssFRRFtR | |
| CAMK2A | Q9UQM7 | human | SCN5A | Q14524 | S577 | tsAQGQPsPGTSAPG | Na_trans_cytopl |
| CAMK2A | Q9UQM7 | human | CTNNB1 | P35222 | S552 | QDtQRRtsMGGtQQQ | |
| CAMK2A | Q9UQM7 | human | DLGAP1 | O14490-2 | S44 | CRRMRSGsYIKAMGD | |
| CAMK2A | Q9UQM7 | human | CREB3L3 | Q68CJ9 | T429 | TLVLRNAtEGLGQVA | |
| CAMK2A | Q9UQM7 | human | SCN5A | Q14524 | S1934 | LFRQQAGsGLsEEDA | |
| CAMK2A | Q9UQM7 | human | SCN5A | Q14524 | S539 | FRRRDLGsEADFADD | Na_trans_cytopl |
| CAMK2A | Q9UQM7 | human | ITGB1 | P05556 | T789 | IyksAVttVVNPkyE | Integrin_b_cyt |
| CAMK2A | Q9UQM7 | human | ALOX5 | P09917 | S272 | CsLERQLsLEQEVQQ | Lipoxygenase |
| CAMK2A | Q9UQM7 | human | SCN5A | Q14524 | S516 | LsLTrGLsRTsMKPR | Na_trans_cytopl |
| CAMK2A | Q9UQM7 | human | ID1 | P41134 | S36 | GEVVRCLsEQSVAIS | |
| CAMK2A | Q9UQM7 | human | SCN5A | Q14524 | S460 | VDtVsRssLEMsPLA | |
| CAMK2A | Q9UQM7 | human | HRH1 | P35367 | S398 | WKRLRsHsRQyVSGL | 7tm_1 |
| CAMK2A | Q9UQM7 | human | ETS1 | P14921 | S257 | DsFEsIEsyDsCDRL | Ets1_N_flank |
| CAMK2A | Q9UQM7 | human | CLCN3 | P51790 | S109 | ERHRRINsKKKESAW | |
| CAMK2A | Q9UQM7 | human | CTNNB1 | P35222 | T332 | VNIMRTytyEkLLWT | |
| CAMK2A | Q9UQM7 | human | SCN8A | Q9UQD0 | S561 | PFLSRHNsKSSIFSF | Na_trans_cytopl |
| CAMK2A | Q9UQM7 | human | IL6ST | P40189 | S782 | QVFSRsEsTQPLLDS | |
| CAMK2A | Q9UQM7 | human | HSF1 | Q00613 | S230 | PkYSRQFsLEHVHGS | |
| CAMK2A | Q9UQM7 | human | HDAC5 | Q9UQL6 | S259 | FPLRkTAsEPNLKVR | |
| CAMK2A | Q9UQM7 | human | CAMK2A | Q9UQM7-2 | T286 | SCMHRQEtVDCLKKF | |
| CAMK2A | Q9UQM7 | human | SMAD2 | Q15796 | S240 | SDQQLNQsMDTGsPA | |
| CAMK2A | Q9UQM7 | human | SCN8A | Q9UQD0 | S641 | RRSVKRNstVDCNGV | Na_trans_cytopl |
| CAMK2A | Q9UQM7 | human | SCN5A | Q14524 | S483 | sKRRKRMssGtEECG | |
| CAMK2A | Q9UQM7 | human | KCND1 | Q9NSA2 | S555 | TASVSRGsMQELDML | |
| CAMK2A | Q9UQM7 | human | FBXO43 | Q4G163 | T234 | FSQQKTStIDDSKDD | |
| CAMK2A | Q9UQM7 | human | CAMK2A | Q9UQM7 | T306 | LkGAILttMLAtRNF | |
| CAMK2A | Q9UQM7 | human | RRAD | P55042 | S273 | AGTRRREsLGKKAKR | |
| CAMK2A | Q9UQM7 | human | TH | P07101-4 | S44 | RFIGRRQsLIEDARK | TOH_N |
| CAMK2A | Q9UQM7 | human | RCHY1 | Q96PM5 | T154 | ICLEDIHtsRVVAHV | zf-RING_2 |
| CAMK2A | Q9UQM7 | human | STMN1 | P16949 | S16 | kELEKrAsGQAFELI | Stathmin |
| CAMK2A | Q9UQM7 | human | PTTG1 | O95997 | S89 | KQKQPsFsAKKMTEK | Securin |
| CAMK2A | Q9UQM7 | human | SCN5A | Q14524 | T17 | TssFRRFtREsLAAI | |
| CAMK2A | Q9UQM7 | human | TH | P07101 | S19 | KGFRRAVsELDAKQA | TOH_N |
| CAMK2A | Q9UQM7 | human | SCN5A | Q14524 | S1920 | RRHLLQRsLKHAsFL | |
| CAMK2A | Q9UQM7 | human | SCN5A | Q14524 | S1885 | KFMAANPsKISYEPI | |
| CAMK2A | Q9UQM7 | human | GRIN2A | Q12879 | S1459 | RVYKKMPsIESDV__ | NMDAR2_C |
| CAMK2A | Q9UQM7 | human | CYLD | Q9NQC7 | S418 | TTENRFHsLPFsLtK | CYLD_phos_site |
| CAMK2A | Q9UQM7 | human | NCOR2 | Q9Y618 | S2453 | VWEDRPssAGstPFP | |
| CAMK2A | Q9UQM7 | human | OPRM1 | P35372 | S268 | LKsVRMLsGsKEKDR | 7tm_1 |
| CAMK2A | Q9UQM7 | human | NOX5 | Q96PH1-4 | S659 | ANKEKKDsITGLQTR | NAD_binding_6 |
| CAMK2A | Q9UQM7 | human | KIF3A | Q9Y496 | S687 | KTGRRKRsAkPEtVI | |
| CAMK2A | Q9UQM7 | human | SCN5A | Q14524 | S667 | QRALsAVsVLtsALE | Na_trans_cytopl |
| CAMK2A | Q9UQM7 | human | DAGLA | Q9Y4D2 | S782 | APLATMEsLSDTESL | |
| CAMK2A | Q9UQM7 | human | SCN5A | Q14524 | S20 | FRRFtREsLAAIEKR | |
| CAMK2A | Q9UQM7 | human | CARD11 | Q9BXL7 | S116 | KEPTRRFstIVVEEG | |
| CAMK2A | Q9UQM7 | human | SCN5A | Q14524 | S1937 | QQAGsGLsEEDAPER | |
| CAMK2A | Q9UQM7 | human | SLC1A3 | P43003 | T37 | KkkVQNItkEDVKSY | |
| CAMK2A | Q9UQM7 | human | PLCB3 | Q01970 | S537 | PSLEPQKsLGDEGLN | |
| CAMK2A | Q9UQM7 | human | PPP2R2B | Q00005-2 | S22 | PNTILsssCHTEADI | |
| CAMK2A | Q9UQM7 | human | RCHY1 | Q96PM5 | S155 | CLEDIHtsRVVAHVL | zf-RING_2 |
| CAMK2A | Q9UQM7 | human | SCN5A | Q14524 | S1865 | TKRVLGEsGEMDALK | |
| CAMK2A | Q9UQM7 | human | SCN5A | Q14524 | S2007 | DLADFPPsPDRDRES | |
| CAMK2A | Q9UQM7 | human | CYLD | Q9NQC7 | S772 | LFKkIFPsLELNITD | UCH |
| CAMK2A | Q9UQM7 | human | CD44 | P16070 | S706 | LNGEAsksQEMVHLV | |
| CAMK2A | Q9UQM7 | human | NOX5 | Q96PH1-4 | S502 | MRKsQRSsKGSEILL | FAD_binding_8 |
| CAMK2A | Q9UQM7 | human | ESPL1 | Q14674 | S1501 | TDNWRKMsFEILRGs | |
| CAMK2A | Q9UQM7 | human | CACNA1H | O95180 | S1198 | tPLRRAEsLDPRPLR | |
| CAMK2A | Q9UQM7 | human | TH | P07101-3 | S40 | RFIGRRQsLIEDARK | TOH_N |
| CAMK2A | Q9UQM7 | human | DLG4 | P78352 | S73 | ITLERGNsGLGFsIA | PDZ |
| CAMK2A | Q9UQM7 | human | SCN5A | Q14524 | S1503 | NAMKKLGsKKPQKPI | |
| CAMK2A | Q9UQM7 | human | BECN1 | Q14457 | S93 | ARMMstEsANsFTLI | |
| CAMK2A | Q9UQM7 | human | SCN5A | Q14524 | S471 | sPLAPVNsHERRsKR | |
| CAMK2A | Q9UQM7 | human | PTTG1 | O95997 | T66 | AtRkALGtVNRATEK | Securin |
| CAMK2A | Q9UQM7 | human | HRH1 | P35367 | S396 | FTWKRLRsHsRQyVS | 7tm_1 |
| CAMK2A | Q9UQM7 | human | ETS2 | P15036 | S246 | FPkSRLSsVsVTYCS | Ets1_N_flank |
| CAMK2A | Q9UQM7 | human | BECN1 | Q14457 | S90 | IPPARMMstEsANsF | |
| CAMK2A | Q9UQM7 | human | SCN5A | Q14524 | S1998 | RGSDYSHsEDLADFP | |
| CAMK2A | Q9UQM7 | human | SCN5A | Q14524 | S1003 | AAQGQLPsCIATPYS | Na_trans_assoc |
| CAMK2A | Q9UQM7 | human | CTNNB1 | P35222 | T472 | ICALRHLtsRHQEAE | Arm |
| CAMK2A | Q9UQM7 | human | GRIN2B | Q13224 | S1303 | NKLRRQHsyDtFVDL | NMDAR2_C |
| CAMK2A | Q9UQM7 | human | GRIA1 | P42261 | S567 | FSPYEWHsEEFEEGR | Lig_chan |
| CAMK2A | Q9UQM7 | human | GLO1 | Q04760 | T107 | ELTHNWGtEDDETQS | Glyoxalase |
| CAMK2A | Q9UQM7 | human | RYR2 | Q92736 | S2808 | YNRTRRIsQTSQVsV | |
| CAMK2A | Q9UQM7 | human | SNCA | P37840 | S129 | NEAyEMPsEEGyQDy | Synuclein |
| CAMK2A | Q9UQM7 | human | MAPT | P10636-8 | S262 | NVKskIGstENLkHQ | Tubulin-binding |
| CAMK2A | Q9UQM7 | human | MAPT | P10636-8 | S416 | sNVsstGsIDMVDsP | |
| CAMK2A | Q9UQM7 | human | HRH1 | P35367 | T140 | LRYLKYRtKtRASAT | 7tm_1 |
| CAMK2A | Q9UQM7 | human | TH | P07101-3 | S19 | KGFRRAVsELDAKQA | TOH_N |
| CAMK2A | Q9UQM7 | human | DAGLA | Q9Y4D2 | S808 | RSIRGsPsLHAVLER | |
| CAMK2A | Q9UQM7 | human | SPTBN4 | Q9H254 | S2254 | RRPERQEsVDQSEEA | |
| CAMK2A | Q9UQM7 | human | PPP2R2B | Q00005-2 | S20 | RPPNTILsssCHTEA | |
| CAMK2A | Q9UQM7 | human | NOX5 | Q96PH1-4 | T494 | KRLsRSVtMRKsQRS | FAD_binding_8 |
| CAMK2A | Q9UQM7 | human | SCN5A | Q14524 | T570 | VPWPLRRtsAQGQPs | Na_trans_cytopl |
| CAMK2A | Q9UQM7 | human | KCNJ11 | Q14654 | T224 | MQVVRKTtSPEGEVV | IRK_C |
| CAMK2A | Q9UQM7 | human | SCN5A | Q14524 | S664 | GARQRALsAVsVLts | Na_trans_cytopl |
| CAMK2A | Q9UQM7 | human | SCN5A | Q14524 | S42 | GSTTLQEsREGLPEE | |
| CAMK2A | Q9UQM7 | human | SCN5A | Q14524 | S499 | DRLPKsDsEDGPRAM | |
| CAMK2A | Q9UQM7 | human | PTTG1 | O95997 | S87 | PLKQKQPsFsAKKMT | Securin |
| CAMK2A | Q9UQM7 | human | PLN | P26678 | T17 | SAIRRAstIEMPQQA | Phospholamban |
| CAMK2A | Q9UQM7 | human | TCAP | O15273 | S161 | RsLsRSMsQEAQRG_ | Telethonin |
| CAMK2A | Q9UQM7 | human | PPP2R2B | Q00005-2 | S21 | PPNTILsssCHTEAD | |
| CAMK2A | Q9UQM7 | human | ETS1 | P14921 | S251 | GkLGGQDsFEsIEsy | Ets1_N_flank |
| CAMK2D | Q13557 | human | HDAC4 | P56524 | S210 | YGKTQHSsLDQSsPP | |
| CAMK2D | Q13557 | human | SCN5A | Q14524 | T594 | LHGkKNStVDCNGVV | Na_trans_cytopl |
| CAMK2D | Q13557 | human | KCNQ1 | P51787 | T482 | sMPHFMRtNsFAEDL | |
| CAMK2D | Q13557 | human | KCNJ11 | Q14654 | T224 | MQVVRKTtSPEGEVV | IRK_C |
| CAMK2D | Q13557 | human | ANKRD28 | O15084 | S1011 | TNTskTVsFEALPIM | |
| CAMK2D | Q13557 | human | RNF8 | O76064 | S157 | LRTKRkFsLDELAGP | |
| CAMK2D | Q13557 | human | TTN | Q8WZ42 | T11922 | EVEVPTVtKRERKIP | |
| CAMK2D | Q13557 | human | CACNB2 | Q08289-2 | T499 | RGLSRQEtFDSETQE | |
| CAMK2D | Q13557 | human | KCNQ1 | P51787 | S484 | PHFMRtNsFAEDLDL | KCNQ_channel |
| CAMK2D | Q13557 | human | CEACAM1 | P13688-8 | S459 | LHFGKtGsSGPLQ__ | |
| CAMK2D | Q13557 | human | TTN | Q8WZ42 | S12022 | RRKLRPGsGGEKPPD | |
| CAMK2D | Q13557 | human | SCN5A | Q14524 | S516 | LsLTrGLsRTsMKPR | Na_trans_cytopl |
| CAMK2D | Q13557 | human | TTN | Q8WZ42 | T11932 | ERKIPEPtKVPEIKP | |
| CAMK2D | Q13557 | human | TTN | Q8WZ42 | T11969 | PPVEPEPtPIAAPVT | |
| CAMK2D | Q13557 | human | CAMK2D | Q13557 | T287 | sMMHRQEtVDCLKKF | |
| CAMK2D | Q13557 | human | OCLN | Q16625 | S471 | LDDyREEsEEyMAAA | Occludin_ELL |
| CAMK2D | Q13557 | human | CAMK2B | Q13554 | T287 | sMMHRQEtVECLKKF | |
| CAMK2D | Q13557 | human | CEACAM1 | P13688-8 | T457 | CFLHFGKtGsSGPLQ | |
| CAMK2D | Q13557 | human | TTN | Q8WZ42 | S11878 | KEEVVLKsVLRKRPE | |
| CAMK2D | Q13557-8 | human | TPD52 | P55327 | S176 | kNsPtFksFEEkVEN | TPD52 |
 Biological Network Integration of This Kinase and Substrates Biological Network Integration of This Kinase and Substrates (GeneMANIA website) |
 Enriched GO biological processes of the phosphorylation target genes of the kinase Enriched GO biological processes of the phosphorylation target genes of the kinase |
| Kinase | GOID | GO term | P.adjust |
| CAMK2A | ID | Description | 0.00e+00 |
| CAMK2A | GO:0006816 | calcium ion transport | 3.58e-08 |
| CAMK2A | GO:0034765 | regulation of monoatomic ion transmembrane transport | 3.58e-08 |
| CAMK2A | GO:0050804 | modulation of chemical synaptic transmission | 5.08e-08 |
| CAMK2A | GO:0099177 | regulation of trans-synaptic signaling | 5.08e-08 |
| CAMK2A | GO:0097553 | calcium ion transmembrane import into cytosol | 5.08e-08 |
| CAMK2A | GO:0048167 | regulation of synaptic plasticity | 6.48e-08 |
| CAMK2A | GO:0070588 | calcium ion transmembrane transport | 6.48e-08 |
| CAMK2A | GO:0042391 | regulation of membrane potential | 7.35e-08 |
| CAMK2A | GO:0009410 | response to xenobiotic stimulus | 6.47e-07 |
| CAMK2A | GO:0010038 | response to metal ion | 7.60e-07 |
| CAMK2A | GO:1902074 | response to salt | 8.59e-07 |
| CAMK2A | GO:0071248 | cellular response to metal ion | 5.61e-06 |
| CAMK2A | GO:0007611 | learning or memory | 6.01e-06 |
| CAMK2A | GO:0071241 | cellular response to inorganic substance | 1.54e-05 |
| CAMK2A | GO:0010959 | regulation of metal ion transport | 1.70e-05 |
| CAMK2A | GO:1904062 | regulation of monoatomic cation transmembrane transport | 1.70e-05 |
| CAMK2A | GO:0032355 | response to estradiol | 1.70e-05 |
| CAMK2A | GO:0050890 | cognition | 1.70e-05 |
| CAMK2A | GO:0007613 | memory | 2.81e-05 |
| CAMK2A | GO:1902075 | cellular response to salt | 5.00e-05 |
| CAMK2A | GO:0043279 | response to alkaloid | 9.45e-05 |
| CAMK2A | GO:0048168 | regulation of neuronal synaptic plasticity | 1.57e-04 |
| CAMK2A | GO:0060047 | heart contraction | 1.96e-04 |
| CAMK2A | GO:0050806 | positive regulation of synaptic transmission | 2.32e-04 |
| CAMK2A | GO:0003015 | heart process | 2.58e-04 |
| CAMK2A | GO:1903522 | regulation of blood circulation | 2.71e-04 |
| CAMK2A | GO:0003012 | muscle system process | 2.87e-04 |
| CAMK2A | GO:0050808 | synapse organization | 4.97e-04 |
| CAMK2A | GO:0006836 | neurotransmitter transport | 6.01e-04 |
| CAMK2A | GO:0008016 | regulation of heart contraction | 6.01e-04 |
| CAMK2A | GO:0019722 | calcium-mediated signaling | 6.01e-04 |
| CAMK2A | GO:0071312 | cellular response to alkaloid | 6.01e-04 |
| CAMK2A | GO:0001666 | response to hypoxia | 6.01e-04 |
| CAMK2A | GO:0098739 | import across plasma membrane | 6.01e-04 |
| CAMK2A | GO:0043270 | positive regulation of monoatomic ion transport | 6.21e-04 |
| CAMK2A | GO:0051090 | regulation of DNA-binding transcription factor activity | 6.90e-04 |
| CAMK2A | GO:0035865 | cellular response to potassium ion | 7.21e-04 |
| CAMK2A | GO:0001508 | action potential | 7.21e-04 |
| CAMK2A | GO:0036293 | response to decreased oxygen levels | 7.79e-04 |
| CAMK2A | GO:0007612 | learning | 7.99e-04 |
| CAMK2A | GO:0042136 | neurotransmitter biosynthetic process | 8.41e-04 |
| CAMK2A | GO:0030902 | hindbrain development | 9.86e-04 |
| CAMK2A | GO:0001504 | neurotransmitter uptake | 9.96e-04 |
| CAMK2A | GO:0022898 | regulation of transmembrane transporter activity | 1.01e-03 |
| CAMK2A | GO:0006813 | potassium ion transport | 1.04e-03 |
| CAMK2A | GO:0050769 | positive regulation of neurogenesis | 1.05e-03 |
| CAMK2A | GO:0061337 | cardiac conduction | 1.10e-03 |
| CAMK2A | GO:0048738 | cardiac muscle tissue development | 1.10e-03 |
| CAMK2A | GO:0090257 | regulation of muscle system process | 1.10e-03 |
Top |
Related Drugs to CAMK2D_CAMK2A |
 Drugs used for this fusion-positive patient. Drugs used for this fusion-positive patient. (Manual curation of PubMed, 04-30-2022 + MyCancerGenome) |
| Hgene | Tgene | Drug | Source | PMID |
 Distribution of the number of studies mentioning CAMK2D-CAMK2A and kinase inhibitors the PubMed Abstract (04-01-2024) Distribution of the number of studies mentioning CAMK2D-CAMK2A and kinase inhibitors the PubMed Abstract (04-01-2024) |
| Fusion gene - drug pair 1 | Fusion gene - drug pair 2 | PMID | Publication date | DOI | Study title |
Top |
Related Diseases to CAMK2D_CAMK2A |
 Diseases that have this fusion gene. Diseases that have this fusion gene. (Manual curation of PubMed, 04-30-2022 + MyCancerGenome) |
| Hgene | Tgene | Disease | Source | PMID |
 Related diseases from the literature mentioned this fusion gene and drug. Related diseases from the literature mentioned this fusion gene and drug. (PubMed, 04-01-2024) |
| MeSH ID | MeSH term |
 Diseases associated with fusion partners. Diseases associated with fusion partners. (DisGeNet 4.0) |
| Partner | Gene | Disease ID | Disease name | # pubmeds | Source |
Top |
Clinical Trials of the Found Drugs/Small Molecules |
 Statistics of the Clinical Trials of the Found Kinase Inibitors from clinicaltrials.gov (06-17-2024) Statistics of the Clinical Trials of the Found Kinase Inibitors from clinicaltrials.gov (06-17-2024) |
 Clinical Trials from clinicaltrials.gov (06-17-2024) Clinical Trials from clinicaltrials.gov (06-17-2024) |
| Fusion Gene | Kinase Inhibitor | NCT ID | Study Status | Phases | Disease | # Enrolment | Date |

