
|
|||||||
|
Fusion Protein:HSP90AB1-MYH11 |
Fusion Gene and Fusion Protein Summary |
 Fusion gene summary Fusion gene summary |
| Fusion partner gene information | Fusion gene name: HSP90AB1-MYH11 | FusionPDB ID: 37804 | FusionGDB2.0 ID: 37804 | Hgene | Tgene | Gene symbol | HSP90AB1 | MYH11 | Gene ID | 3326 | 4629 |
| Gene name | heat shock protein 90 alpha family class B member 1 | myosin heavy chain 11 | |
| Synonyms | D6S182|HSP84|HSP90B|HSPC2|HSPCB | AAT4|FAA4|SMHC|SMMHC | |
| Cytomap | 6p21.1 | 16p13.11 | |
| Type of gene | protein-coding | protein-coding | |
| Description | heat shock protein HSP 90-betaHSP90-betaheat shock 84 kDaheat shock 90kD protein 1, betaheat shock protein 90 kDaheat shock protein 90kDa alpha (cytosolic), class B member 1heat shock protein 90kDa alpha family class B member 1 | myosin-11epididymis secretory sperm binding proteinmyosin heavy chain, smooth muscle isoformmyosin, heavy chain 11, smooth musclemyosin, heavy polypeptide 11, smooth muscle | |
| Modification date | 20200327 | 20200322 | |
| UniProtAcc | P08238 Main function of 5'-partner protein: FUNCTION: Molecular chaperone that promotes the maturation, structural maintenance and proper regulation of specific target proteins involved for instance in cell cycle control and signal transduction. Undergoes a functional cycle linked to its ATPase activity. This cycle probably induces conformational changes in the client proteins, thereby causing their activation. Interacts dynamically with various co-chaperones that modulate its substrate recognition, ATPase cycle and chaperone function (PubMed:16478993, PubMed:19696785). Engages with a range of client protein classes via its interaction with various co-chaperone proteins or complexes, that act as adapters, simultaneously able to interact with the specific client and the central chaperone itself. Recruitment of ATP and co-chaperone followed by client protein forms a functional chaperone. After the completion of the chaperoning process, properly folded client protein and co-chaperone leave HSP90 in an ADP-bound partially open conformation and finally, ADP is released from HSP90 which acquires an open conformation for the next cycle (PubMed:27295069, PubMed:26991466). Apart from its chaperone activity, it also plays a role in the regulation of the transcription machinery. HSP90 and its co-chaperones modulate transcription at least at three different levels. They first alter the steady-state levels of certain transcription factors in response to various physiological cues. Second, they modulate the activity of certain epigenetic modifiers, such as histone deacetylases or DNA methyl transferases, and thereby respond to the change in the environment. Third, they participate in the eviction of histones from the promoter region of certain genes and thereby turn on gene expression (PubMed:25973397). Antagonizes STUB1-mediated inhibition of TGF-beta signaling via inhibition of STUB1-mediated SMAD3 ubiquitination and degradation (PubMed:24613385). Promotes cell differentiation by chaperoning BIRC2 and thereby protecting from auto-ubiquitination and degradation by the proteasomal machinery (PubMed:18239673). Main chaperone involved in the phosphorylation/activation of the STAT1 by chaperoning both JAK2 and PRKCE under heat shock and in turn, activates its own transcription (PubMed:20353823). Involved in the translocation into ERGIC (endoplasmic reticulum-Golgi intermediate compartment) of leaderless cargos (lacking the secretion signal sequence) such as the interleukin 1/IL-1; the translocation process is mediated by the cargo receptor TMED10 (PubMed:32272059). {ECO:0000269|PubMed:16478993, ECO:0000269|PubMed:18239673, ECO:0000269|PubMed:19696785, ECO:0000269|PubMed:20353823, ECO:0000269|PubMed:24613385, ECO:0000269|PubMed:32272059, ECO:0000303|PubMed:25973397, ECO:0000303|PubMed:26991466, ECO:0000303|PubMed:27295069}. | P35749 Main function of 5'-partner protein: FUNCTION: Muscle contraction. | |
| Ensembl transtripts involved in fusion gene | ENST ids | ENST00000353801, ENST00000371554, ENST00000371646, | ENST00000573908, ENST00000300036, ENST00000396324, ENST00000452625, ENST00000576790, |
| Fusion gene scores for assessment (based on all fusion genes of FusionGDB 2.0) | * DoF score | 20 X 20 X 7=2800 | 44 X 55 X 10=24200 |
| # samples | 22 | 62 | |
| ** MAII score | log2(22/2800*10)=-3.66985139830767 possibly effective Gene in Pan-Cancer Fusion Genes (peGinPCFGs). DoF>8 and MAII<0 | log2(62/24200*10)=-5.28659502177508 possibly effective Gene in Pan-Cancer Fusion Genes (peGinPCFGs). DoF>8 and MAII<0 | |
| Fusion gene context | PubMed: HSP90AB1 [Title/Abstract] AND MYH11 [Title/Abstract] AND fusion [Title/Abstract] | ||
| Fusion neoantigen context | PubMed: HSP90AB1 [Title/Abstract] AND MYH11 [Title/Abstract] AND neoantigen [Title/Abstract] | ||
| Most frequent breakpoint (based on all fusion genes of FusionGDB 2.0) | HSP90AB1(44218074)-MYH11(15815477), # samples:1 | ||
| Anticipated loss of major functional domain due to fusion event. | HSP90AB1-MYH11 seems lost the major protein functional domain in Hgene partner, which is a CGC by not retaining the major functional domain in the partially deleted in-frame ORF. HSP90AB1-MYH11 seems lost the major protein functional domain in Hgene partner, which is a CGC by not retaining the major functional domain in the partially deleted in-frame ORF. HSP90AB1-MYH11 seems lost the major protein functional domain in Hgene partner, which is a essential gene by not retaining the major functional domain in the partially deleted in-frame ORF. HSP90AB1-MYH11 seems lost the major protein functional domain in Hgene partner, which is a essential gene by not retaining the major functional domain in the partially deleted in-frame ORF. | ||
| * DoF score (Degree of Frequency) = # partners X # break points X # cancer types ** MAII score (Major Active Isofusion Index) = log2(# samples/DoF score*10) |
 Gene ontology of each fusion partner gene with evidence of Inferred from Direct Assay (IDA) from Entrez Gene ontology of each fusion partner gene with evidence of Inferred from Direct Assay (IDA) from Entrez |
| Partner | Gene | GO ID | GO term | PubMed ID |
| Hgene | HSP90AB1 | GO:0007004 | telomere maintenance via telomerase | 10197982 |
| Hgene | HSP90AB1 | GO:0030511 | positive regulation of transforming growth factor beta receptor signaling pathway | 24613385 |
| Hgene | HSP90AB1 | GO:0031396 | regulation of protein ubiquitination | 16809764 |
| Hgene | HSP90AB1 | GO:0032435 | negative regulation of proteasomal ubiquitin-dependent protein catabolic process | 24613385 |
| Hgene | HSP90AB1 | GO:0032516 | positive regulation of phosphoprotein phosphatase activity | 26593036 |
| Hgene | HSP90AB1 | GO:0051131 | chaperone-mediated protein complex assembly | 10811660 |
| Hgene | HSP90AB1 | GO:0051973 | positive regulation of telomerase activity | 10197982 |
| Hgene | HSP90AB1 | GO:1901389 | negative regulation of transforming growth factor beta activation | 20599762 |
| Hgene | HSP90AB1 | GO:1905323 | telomerase holoenzyme complex assembly | 10197982 |
| Hgene | HSP90AB1 | GO:2000010 | positive regulation of protein localization to cell surface | 23431407 |
 Four levels of functional features of fusion genes Four levels of functional features of fusion genesGo to FGviewer search page for the most frequent breakpoint (https://ccsmweb.uth.edu/FGviewer/chr6:44218074/chr16:15815477) - FGviewer provides the online visualization of the retention search of the protein functional features across DNA, RNA, protein, and pathological levels. - How to search 1. Put your fusion gene symbol. 2. Press the tab key until there will be shown the breakpoint information filled. 4. Go down and press 'Search' tab twice. 4. Go down to have the hyperlink of the search result. 5. Click the hyperlink. 6. See the FGviewer result for your fusion gene. |
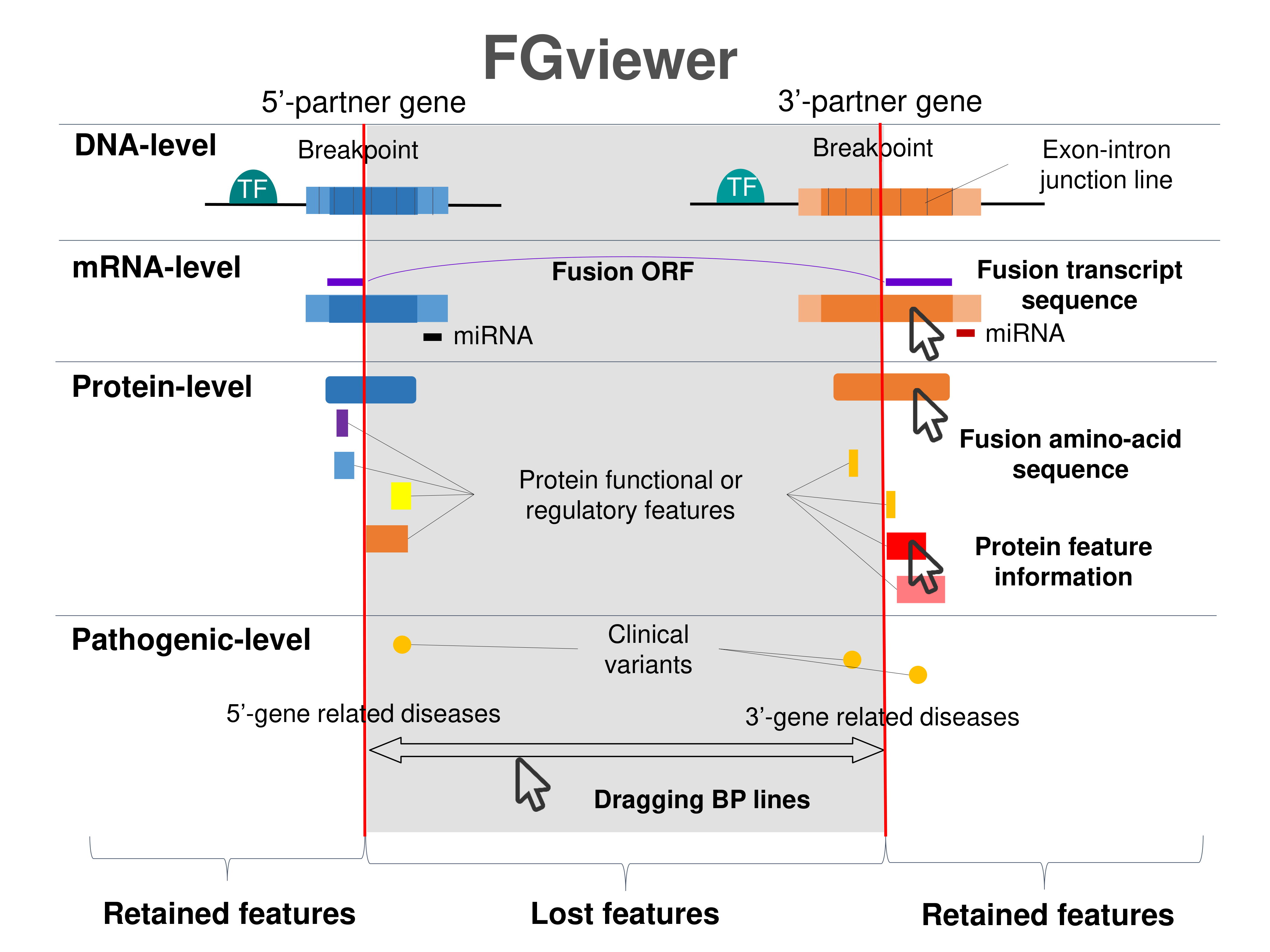 |
 Retention analysis results of each fusion partner protein across 39 protein features of UniProt such as six molecule processing features, 13 region features, four site features, six amino acid modification features, two natural variation features, five experimental info features, and 3 secondary structure features, are available here. Retention analysis results of each fusion partner protein across 39 protein features of UniProt such as six molecule processing features, 13 region features, four site features, six amino acid modification features, two natural variation features, five experimental info features, and 3 secondary structure features, are available here. |
 Fusion gene breakpoints across HSP90AB1 (5'-gene) Fusion gene breakpoints across HSP90AB1 (5'-gene)* Click on the image to open the UCSC genome browser with custom track showing this image in a new window. |
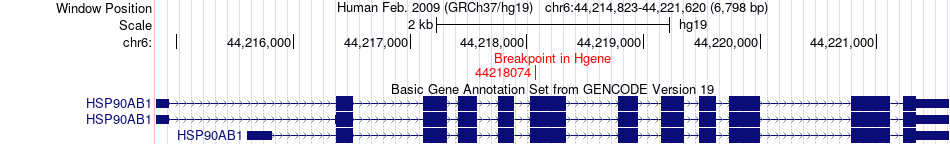 |
 Fusion gene breakpoints across MYH11 (3'-gene) Fusion gene breakpoints across MYH11 (3'-gene)* Click on the image to open the UCSC genome browser with custom track showing this image in a new window. |
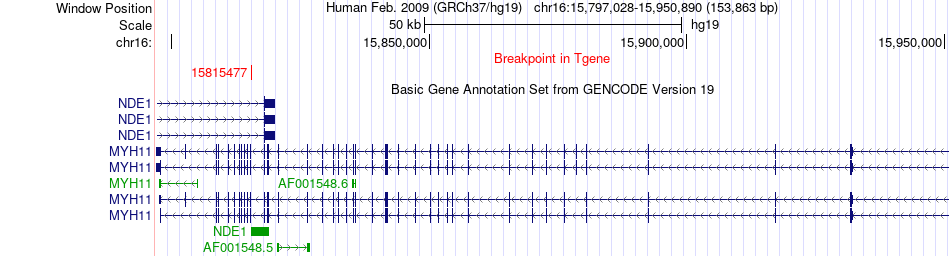 |
Top |
Fusion Amino Acid Sequences |
 Fusion information from ORFfinder translation from full-length transcript sequence from FusionPDB. Fusion information from ORFfinder translation from full-length transcript sequence from FusionPDB. |
| Henst | Tenst | Hgene | Hchr | Hbp | Hstrand | Tgene | Tchr | Tbp | Tstrand | Seq length (transcript) | BP loci (transcript) | Predicted start (transcript) | Predicted stop (transcript) | Seq length (amino acids) |
| ENST00000353801 | HSP90AB1 | chr6 | 44218074 | + | ENST00000396324 | MYH11 | chr16 | 15815477 | - | 3391 | 1032 | 2796 | 1906 | 296 |
| ENST00000353801 | HSP90AB1 | chr6 | 44218074 | + | ENST00000452625 | MYH11 | chr16 | 15815477 | - | 3430 | 1032 | 2835 | 1906 | 309 |
| ENST00000353801 | HSP90AB1 | chr6 | 44218074 | + | ENST00000576790 | MYH11 | chr16 | 15815477 | - | 2815 | 1032 | 2231 | 1173 | 352 |
| ENST00000353801 | HSP90AB1 | chr6 | 44218074 | + | ENST00000300036 | MYH11 | chr16 | 15815477 | - | 2572 | 1032 | 1988 | 918 | 356 |
| ENST00000371646 | HSP90AB1 | chr6 | 44218074 | + | ENST00000396324 | MYH11 | chr16 | 15815477 | - | 3378 | 1019 | 2783 | 1893 | 296 |
| ENST00000371646 | HSP90AB1 | chr6 | 44218074 | + | ENST00000452625 | MYH11 | chr16 | 15815477 | - | 3417 | 1019 | 2822 | 1893 | 309 |
| ENST00000371646 | HSP90AB1 | chr6 | 44218074 | + | ENST00000576790 | MYH11 | chr16 | 15815477 | - | 2802 | 1019 | 2218 | 1160 | 352 |
| ENST00000371646 | HSP90AB1 | chr6 | 44218074 | + | ENST00000300036 | MYH11 | chr16 | 15815477 | - | 2559 | 1019 | 1975 | 905 | 356 |
| ENST00000371554 | HSP90AB1 | chr6 | 44218074 | + | ENST00000396324 | MYH11 | chr16 | 15815477 | - | 3483 | 1124 | 2888 | 1998 | 296 |
| ENST00000371554 | HSP90AB1 | chr6 | 44218074 | + | ENST00000452625 | MYH11 | chr16 | 15815477 | - | 3522 | 1124 | 2927 | 1998 | 309 |
| ENST00000371554 | HSP90AB1 | chr6 | 44218074 | + | ENST00000576790 | MYH11 | chr16 | 15815477 | - | 2907 | 1124 | 2323 | 1265 | 352 |
| ENST00000371554 | HSP90AB1 | chr6 | 44218074 | + | ENST00000300036 | MYH11 | chr16 | 15815477 | - | 2664 | 1124 | 2080 | 1010 | 356 |
 DeepORF prediction of the coding potential based on the fusion transcript sequence of in-frame fusion genes. DeepORF is a coding potential classifier based on convolutional neural network by comparing the real Ribo-seq data. If the no-coding score < 0.5 and coding score > 0.5, then the in-frame fusion transcript is predicted as being likely translated. DeepORF prediction of the coding potential based on the fusion transcript sequence of in-frame fusion genes. DeepORF is a coding potential classifier based on convolutional neural network by comparing the real Ribo-seq data. If the no-coding score < 0.5 and coding score > 0.5, then the in-frame fusion transcript is predicted as being likely translated. |
| Henst | Tenst | Hgene | Hchr | Hbp | Hstrand | Tgene | Tchr | Tbp | Tstrand | No-coding score | Coding score |
| ENST00000353801 | ENST00000396324 | HSP90AB1 | chr6 | 44218074 | + | MYH11 | chr16 | 15815477 | - | 0.004085081 | 0.9959149 |
| ENST00000353801 | ENST00000452625 | HSP90AB1 | chr6 | 44218074 | + | MYH11 | chr16 | 15815477 | - | 0.001415774 | 0.9985843 |
| ENST00000353801 | ENST00000576790 | HSP90AB1 | chr6 | 44218074 | + | MYH11 | chr16 | 15815477 | - | 0.001248787 | 0.99875116 |
| ENST00000353801 | ENST00000300036 | HSP90AB1 | chr6 | 44218074 | + | MYH11 | chr16 | 15815477 | - | 0.003294026 | 0.99670595 |
| ENST00000371646 | ENST00000396324 | HSP90AB1 | chr6 | 44218074 | + | MYH11 | chr16 | 15815477 | - | 0.004101263 | 0.99589866 |
| ENST00000371646 | ENST00000452625 | HSP90AB1 | chr6 | 44218074 | + | MYH11 | chr16 | 15815477 | - | 0.001440065 | 0.9985599 |
| ENST00000371646 | ENST00000576790 | HSP90AB1 | chr6 | 44218074 | + | MYH11 | chr16 | 15815477 | - | 0.00149371 | 0.99850625 |
| ENST00000371646 | ENST00000300036 | HSP90AB1 | chr6 | 44218074 | + | MYH11 | chr16 | 15815477 | - | 0.002904587 | 0.99709547 |
| ENST00000371554 | ENST00000396324 | HSP90AB1 | chr6 | 44218074 | + | MYH11 | chr16 | 15815477 | - | 0.003949659 | 0.9960503 |
| ENST00000371554 | ENST00000452625 | HSP90AB1 | chr6 | 44218074 | + | MYH11 | chr16 | 15815477 | - | 0.001294425 | 0.9987056 |
| ENST00000371554 | ENST00000576790 | HSP90AB1 | chr6 | 44218074 | + | MYH11 | chr16 | 15815477 | - | 0.001448386 | 0.99855155 |
| ENST00000371554 | ENST00000300036 | HSP90AB1 | chr6 | 44218074 | + | MYH11 | chr16 | 15815477 | - | 0.00282304 | 0.997177 |
 Predicted full-length fusion amino acid sequences. For individual full-length fusion transcript sequence from FusionPDB, we ran ORFfinder and chose the longest ORF among all the predicted ones. Predicted full-length fusion amino acid sequences. For individual full-length fusion transcript sequence from FusionPDB, we ran ORFfinder and chose the longest ORF among all the predicted ones. |
Get the fusion protein sequences from here. |
| Fusion protein sequence information is available in the fasta format. >FusionGDB ID_FusionGDB isoform ID_FGname_Hgene_Hchr_Hbp_Henst_Tgene_Tchr_Tbp_Tenst_length(fusion AA) seq_BP |
Top |
Fusion Protein Breakpoint Sequences for HSP90AB1-MYH11 |
 +/-13 AA sequence from the breakpoints of the fusion protein sequences. +/-13 AA sequence from the breakpoints of the fusion protein sequences. |
| Hgene | Hchr | Hbp | Tgene | Tchr | Tbp | Length(fusion protein) | BP in fusion protein | Peptide |
| HSP90AB1 | chr6 | 44218074 | MYH11 | chr16 | 15815477 | 1019 | 36 | GHLPALFESPSSEPSRQATGQLLCQF |
| HSP90AB1 | chr6 | 44218074 | MYH11 | chr16 | 15815477 | 1032 | 36 | GHLPALFESPSSEPSRQATGQLLCQF |
| HSP90AB1 | chr6 | 44218074 | MYH11 | chr16 | 15815477 | 1124 | 36 | GHLPALFESPSSEPSRQATGQLLCQF |
Top |
Potential FusionNeoAntigen Information of HSP90AB1-MYH11 in HLA I |
 Multiple sequence alignments of the potential FusionNeoAntigens per fusion breakpoints. If the MSA is empty, then it means that there were predicted fusion neoantigens in this fusion breakpoint, but those predicted fusion neoantigens were not across the breakpoint, which is not fusion-specific. Multiple sequence alignments of the potential FusionNeoAntigens per fusion breakpoints. If the MSA is empty, then it means that there were predicted fusion neoantigens in this fusion breakpoint, but those predicted fusion neoantigens were not across the breakpoint, which is not fusion-specific. |
 Potential FusionNeoAntigen Information Potential FusionNeoAntigen Information* We used NetMHCpan v4.1 (%rank<0.5) and deepHLApan v1.1 (immunogenic score>0.5) |
| Fusion gene | Hchr | Hbp | Tgene | Tchr | Tbp | HLA I | FusionNeoAntigen peptide | Binding score | Immunogenic score | Neoantigen start (at BP 13) | Neoantigen end (at BP 13) |
Top |
Potential FusionNeoAntigen Information of HSP90AB1-MYH11 in HLA II |
 Multiple sequence alignments of the potential FusionNeoAntigens per fusion breakpoints. If the MSA is empty, then it means that there were predicted fusion neoantigens in this fusion breakpoint, but those predicted fusion neoantigens were not across the breakpoint, which is not fusion-specific. Multiple sequence alignments of the potential FusionNeoAntigens per fusion breakpoints. If the MSA is empty, then it means that there were predicted fusion neoantigens in this fusion breakpoint, but those predicted fusion neoantigens were not across the breakpoint, which is not fusion-specific. |
 Potential FusionNeoAntigen Information Potential FusionNeoAntigen Information * We used NetMHCIIpan v4.1 (%rank<0.5). |
| Fusion gene | Hchr | Hbp | Tgene | Tchr | Tbp | HLA II | FusionNeoAntigen peptide | Neoantigen start (at BP 13) | Neoantigen end (at BP 13) |
Top |
Fusion breakpoint peptide structures of HSP90AB1-MYH11 |
 3D structures of the fusion breakpoint peptide of 14AA sequence that have potential fusion neoantigens 3D structures of the fusion breakpoint peptide of 14AA sequence that have potential fusion neoantigens* The minimum length of the amino acid sequence in RoseTTAFold is 14AA. Here, we predicted the 14AA fusion protein breakpoint sequence not the fusion neoantigen peptide, which is shorter than 14 AA. |
Top |
Filtering FusionNeoAntigens Through Checking the Interaction with HLAs in 3D of HSP90AB1-MYH11 |
 Virtual screening between 25 HLAs (from PDB) and FusionNeoAntigens Virtual screening between 25 HLAs (from PDB) and FusionNeoAntigens* We used Glide to predict the interaction between HLAs and neoantigens. |
| HLA allele | PDB ID | File name | BPseq | Docking score | Glide score |
Top |
Vaccine Design for the FusionNeoAntigens of HSP90AB1-MYH11 |
 mRNA and peptide sequences of FusionNeoAntigens that have potential interaction with HLA-Is. mRNA and peptide sequences of FusionNeoAntigens that have potential interaction with HLA-Is. |
| Fusion gene | Hchr | Hbp | Tchr | Tbp | Start in +/-13AA | End in +/-13AA | FusionNeoAntigen peptide sequence | FusionNeoAntigen RNA sequence |
 mRNA and peptide sequences of FusionNeoAntigens that have potential interaction with HLA-IIs. mRNA and peptide sequences of FusionNeoAntigens that have potential interaction with HLA-IIs. |
| Fusion gene | Hchr | Hbp | Tchr | Tbp | Start in +/-13AA | End in +/-13AA | FusionNeoAntigen peptide | FusionNEoAntigen RNA sequence |
Top |
Information of the samples that have these potential fusion neoantigens of HSP90AB1-MYH11 |
 These samples were reported as having these fusion breakpoints. For individual breakpoints, we checked the open reading frames considering multiple gene isoforms and chose the in-frame fusion genes only. Then, we made fusion protein sequences and predicted the fusion neoantigens. These fusion-positive samples may have these potential fusion neoantigens. These samples were reported as having these fusion breakpoints. For individual breakpoints, we checked the open reading frames considering multiple gene isoforms and chose the in-frame fusion genes only. Then, we made fusion protein sequences and predicted the fusion neoantigens. These fusion-positive samples may have these potential fusion neoantigens. |
| Cancer type | Fusion gene | Hchr | Hbp | Henst | Tchr | Tbp | Tenst | Sample |
Top |
Potential target of CAR-T therapy development for HSP90AB1-MYH11 |
 Predicted 3D structure. We used RoseTTAFold. Predicted 3D structure. We used RoseTTAFold. |
 Retention analysis result of each fusion partner protein across 39 protein features of UniProt such as six molecule processing features, 13 region features, four site features, six amino acid modification features, two natural variation features, five experimental info features, and 3 secondary structure features. Here, to provide the retention of the transmembrane domain, we only show the protein feature retention information of those transmembrane features Retention analysis result of each fusion partner protein across 39 protein features of UniProt such as six molecule processing features, 13 region features, four site features, six amino acid modification features, two natural variation features, five experimental info features, and 3 secondary structure features. Here, to provide the retention of the transmembrane domain, we only show the protein feature retention information of those transmembrane features* Minus value of BPloci means that the break point is located before the CDS. |
| - In-frame and retained 'Transmembrane'. |
| Partner | Gene | Hbp | Tbp | ENST | Strand | BPexon | TotalExon | Protein feature loci | *BPloci | TotalLen | Protein feature | Protein feature note |
 Subcellular localization prediction of the transmembrane domain retained fusion proteins Subcellular localization prediction of the transmembrane domain retained fusion proteins* We used DeepLoc 1.0. The order of the X-axis of the barplot is as follows: Entry_ID, Localization, Type, Nucleus, Cytoplasm, Extracellular, Mitochondrion, Cell_membrane, Endoplasmic_reticulum, Plastid, Golgi.apparatus, Lysosome.Vacuole, Peroxisome. Y-axis is the output score of DeepLoc. Clicking the image will open a new tab with a large image. |
| Hgene | Hchr | Hbp | Henst | Tgene | Tchr | Tbp | Tenst | DeepLoc result |
Top |
Related Drugs to HSP90AB1-MYH11 |
 Drugs used for this fusion-positive patient. Drugs used for this fusion-positive patient. (Manual curation of PubMed, 04-30-2022 + MyCancerGenome) |
| Hgene | Tgene | Drug | Source | PMID |
Top |
Related Diseases to HSP90AB1-MYH11 |
 Diseases that have this fusion gene. Diseases that have this fusion gene. (Manual curation of PubMed, 04-30-2022 + MyCancerGenome) |
| Hgene | Tgene | Disease | Source | PMID |
 Diseases associated with fusion partners. Diseases associated with fusion partners. (DisGeNet 4.0) |
| Partner | Gene | Disease ID | Disease name | # pubmeds | Source |
| Tgene | MYH11 | C4707243 | Familial thoracic aortic aneurysm and aortic dissection | 10 | CLINGEN;GENOMICS_ENGLAND |
| Tgene | MYH11 | C0023467 | Leukemia, Myelocytic, Acute | 2 | CTD_human |
| Tgene | MYH11 | C0023479 | Acute myelomonocytic leukemia | 2 | CTD_human;ORPHANET |
| Tgene | MYH11 | C0026998 | Acute Myeloid Leukemia, M1 | 2 | CTD_human |
| Tgene | MYH11 | C1851504 | Aortic aneurysm, familial thoracic 4 | 2 | CTD_human;GENOMICS_ENGLAND;UNIPROT |
| Tgene | MYH11 | C1879321 | Acute Myeloid Leukemia (AML-M2) | 2 | CTD_human |
| Tgene | MYH11 | C1608393 | Megacystis microcolon intestinal hypoperistalsis syndrome | 1 | GENOMICS_ENGLAND;ORPHANET |

